- Joined
- Jul 6, 2009
- Messages
- 582
- Reaction score
- 481
There is another thread on the forum discussing a new automated Alkalinity monitor/controller https://www.reef2reef.com/threads/would-you-invest-a-grand-for-a-kh-guardian.276668/
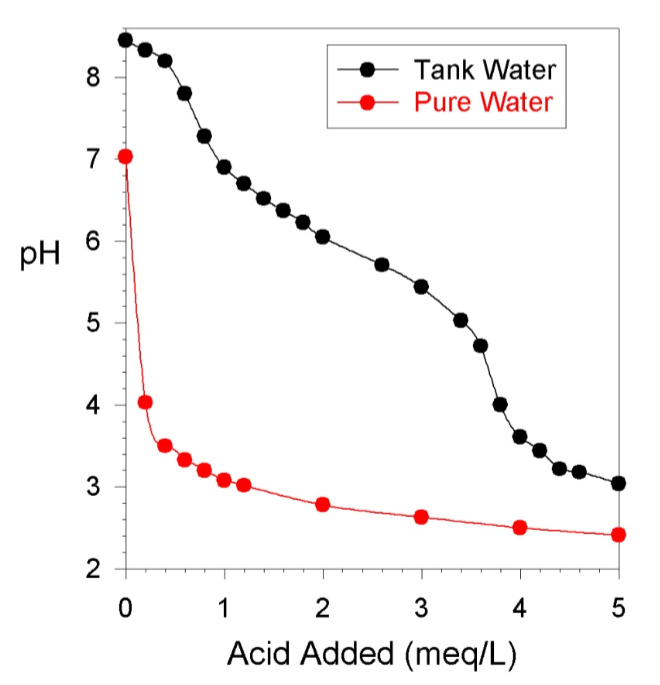
. The premise of the unit is the ability to calculate Alk from Ph. I would like to know how this works given Ph swings in our aquarium with constant Alk given Co2 swings ext?
. The premise of the unit is the ability to calculate Alk from Ph. I would like to know how this works given Ph swings in our aquarium with constant Alk given Co2 swings ext?