Any help identifying what this is?

Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Microscope ID
- Thread starter Reefer911
- Start date
- Tagged users None
Detritus, probably. What magnification and what kind of things are you looking for? If you're looking for dinos or plankton, a higher magnification and some finer focus on the smaller round dots (big masses tend to be some kind of detritus or collected stuff) should help.
I’ll try to get a better slide. Lots of browning on the sand
This is at 1000x magnification. At this power, I thought I would get a better view.
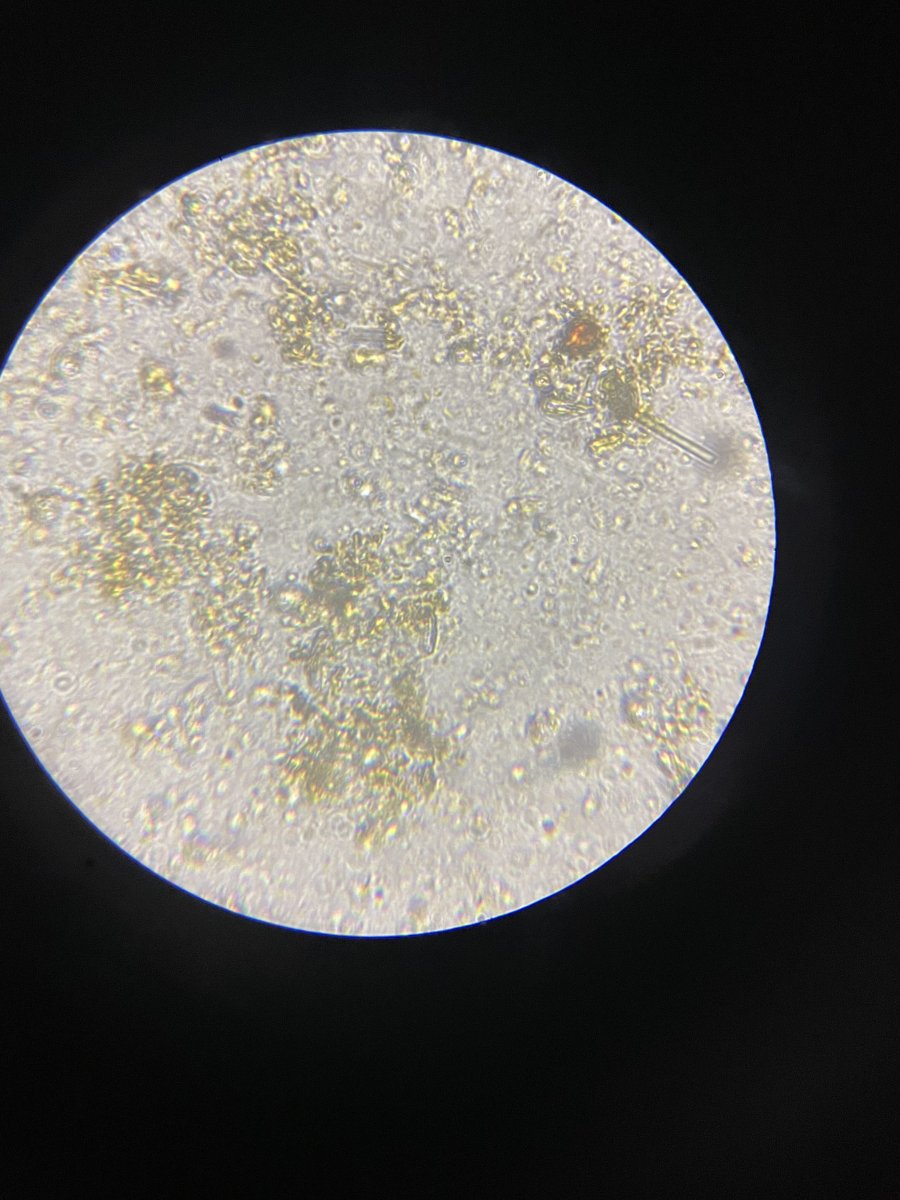
Are you using a cover slip? Have you tried wicking away some of the water from the corner of the cover slip with a bit of paper towel?
1000x is probably overkill for what's necessary to see it, maybe 300-600x would be plenty, but the issue you may be running into is trying to find what you're looking for in a thick sample. For any objective, but especially so for high magnification, the depth of the focal plane is very shallow, so getting the sample as close to a single layer sandwiched between the slide and the cover slip should make it easier to find focus on what you're looking at. Also worth mentioning, if your objective used is an oil or water immersion lens, a drop of that medium on top of the coverslip (in contact with the microscope objective's lens when in use) should help get some sharpness and detail in the image.
Since this sounds like a diatom/dinoflagellate sample of some description, you could try getting a small gob of it and sort of mashing it into the slide, removing as many chunks as possible. The goal isn't to damage it (and that's probably not likely), but to make a very thin smear of the thing you're looking at - if the object is several layers thick, even in focus parts will have fuzz around from the out of focus parts too.
You can also try slowly moving through the focus to look at specific areas - the boundary of an object should go from fuzzy with an external perimeter to sharp to fuzzy with an internal perimeter, so if you pick a specific object and try to find that sharp point between the two, you can get the specific region of interest in sharpest focus.
Finally, changing the amount of light can improve contrast when you're in focus. You'd think more light would just be better, but there does seem to be a happy medium that lets you visualize the contrast that's there better.
From the image you have here, the round particles are some kind of small plankton and the geometric looking parts are probably diatoms. From the size, they are likely dinoflagellates - I'd expect diatoms to be mostly those more geometric looking shapes (they can be circular, though), and I'd expect cyanobacteria to look like chains of little round balls.
1000x is probably overkill for what's necessary to see it, maybe 300-600x would be plenty, but the issue you may be running into is trying to find what you're looking for in a thick sample. For any objective, but especially so for high magnification, the depth of the focal plane is very shallow, so getting the sample as close to a single layer sandwiched between the slide and the cover slip should make it easier to find focus on what you're looking at. Also worth mentioning, if your objective used is an oil or water immersion lens, a drop of that medium on top of the coverslip (in contact with the microscope objective's lens when in use) should help get some sharpness and detail in the image.
Since this sounds like a diatom/dinoflagellate sample of some description, you could try getting a small gob of it and sort of mashing it into the slide, removing as many chunks as possible. The goal isn't to damage it (and that's probably not likely), but to make a very thin smear of the thing you're looking at - if the object is several layers thick, even in focus parts will have fuzz around from the out of focus parts too.
You can also try slowly moving through the focus to look at specific areas - the boundary of an object should go from fuzzy with an external perimeter to sharp to fuzzy with an internal perimeter, so if you pick a specific object and try to find that sharp point between the two, you can get the specific region of interest in sharpest focus.
Finally, changing the amount of light can improve contrast when you're in focus. You'd think more light would just be better, but there does seem to be a happy medium that lets you visualize the contrast that's there better.
From the image you have here, the round particles are some kind of small plankton and the geometric looking parts are probably diatoms. From the size, they are likely dinoflagellates - I'd expect diatoms to be mostly those more geometric looking shapes (they can be circular, though), and I'd expect cyanobacteria to look like chains of little round balls.
Yes I do have cover slips. I placed the sample in the slide with as small amount of water as I could and put the cover slip over it, then smeared it around to try to thin out the sample. This is a cheap amscope so no oil immersion lens.
I’ll try to see if I can get it any better and post back
I’ll try to see if I can get it any better and post back
This is 400x but I zoomed in and saved that. Still not looking as good as some of the other pics I’ve seen. This scope doesn’t have a fine tuning focus. I wish I could get a better pic
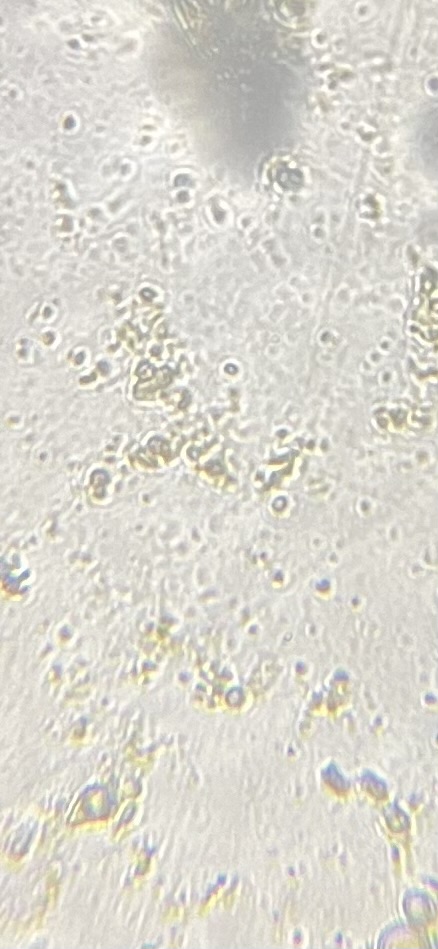
Does it feel like there's any slop in the focus knob? As in, you get it to in focus and when you let go it moves? If so, there is usually a tension adjustment screw that could help keep it in place. In that image, you can see the dark spots with a little bit of white halo - that's the external ring I mentioned in focusing. The circular spot lower center of the image is probably more in focus for the dino shaped things, but the ones right in the center of the image are not quite in focus (when focusing, that white halo should contract to the boundary of the object, then passing through the other side of focus, the interior of it should. It does look to me like the amount of material on the sample and its overall thickness is much decreased, though, so it's probably easier to find the point where things start looking reasonable.
I would say keep trying, and make sure that you can identify when the focus is on the top of the cover slip and on the top of the slide (more as a diagnostic and to get an idea what you're working with), but this is more or less what I'd expect with a cheap microscope. If you've got an iris to adjust light, that could be helpful, and again if there's an immersion medium for your higher power objectives, that can help, but it may be just try a few attempts until you get a good sample in a good configuration and have a feel for focusing. While perhaps obvious, I would try to focus your efforts on the center of the image in terms of focusing and adjustment, it looks like off-axis areas (like the bottom of the image) are showing a fair bit of chromatic aberration which will reduce how sharp something can look. Depending on your camera setup, the camera may be at a slightly different focus point than your eye, so it's probably best to do the final focusing through the camera's view if possible.
Another potential option to increase contrast would be staining - and both Lugols Iodine and Methelyene blue are used for fish/coral treatment and supplementation, but also staining samples for microscopy. Color shouldn't matter too much for ID (it's just going to be slightly greenish brown), so the extra contrast from a stain could help bring out form and edges.
I would say keep trying, and make sure that you can identify when the focus is on the top of the cover slip and on the top of the slide (more as a diagnostic and to get an idea what you're working with), but this is more or less what I'd expect with a cheap microscope. If you've got an iris to adjust light, that could be helpful, and again if there's an immersion medium for your higher power objectives, that can help, but it may be just try a few attempts until you get a good sample in a good configuration and have a feel for focusing. While perhaps obvious, I would try to focus your efforts on the center of the image in terms of focusing and adjustment, it looks like off-axis areas (like the bottom of the image) are showing a fair bit of chromatic aberration which will reduce how sharp something can look. Depending on your camera setup, the camera may be at a slightly different focus point than your eye, so it's probably best to do the final focusing through the camera's view if possible.
Another potential option to increase contrast would be staining - and both Lugols Iodine and Methelyene blue are used for fish/coral treatment and supplementation, but also staining samples for microscopy. Color shouldn't matter too much for ID (it's just going to be slightly greenish brown), so the extra contrast from a stain could help bring out form and edges.
The knob is tight. It doesn’t feel loose at all, and the focus stays where I set it.
I haven’t stained anything since microbiology 20+ years ago. If I can get some methylene blue (I work in a hospital) how would I go about doing that?
I haven’t stained anything since microbiology 20+ years ago. If I can get some methylene blue (I work in a hospital) how would I go about doing that?
Haven't done a lot of fixing for mine, but it looks like methanol fixing is a preferred method:

 www.protocols.io
www.protocols.io
That said, I'd probably try just mixing a drop of it with a mL or two of your sample, waiting a couple minutes, and then mounting a sample of that on the slide as a starting point. Since it's a dye which is absorbed by the cells, once you wait a bit, you can probably dilute the remainder in the water quite a bit, maybe 10x the volume (I would use fresh saltwater to keep cells from bursting because of osmotic pressure), then decant off the water part down the drain when the bits have settled. I'd try the same procedure if you were using Lugols as well as a starting point.

Methylene Blue staining
A simple protocol for staining with Methylene Blue, a very commonly available stain.
That said, I'd probably try just mixing a drop of it with a mL or two of your sample, waiting a couple minutes, and then mounting a sample of that on the slide as a starting point. Since it's a dye which is absorbed by the cells, once you wait a bit, you can probably dilute the remainder in the water quite a bit, maybe 10x the volume (I would use fresh saltwater to keep cells from bursting because of osmotic pressure), then decant off the water part down the drain when the bits have settled. I'd try the same procedure if you were using Lugols as well as a starting point.
I think I’m going to return this microscope since it doesn’t have a fine focus knob. Didn’t realize that and it’s hard to focus with the coarse focus.
It came with a camera so maybe that’s where the fine focus can be done but I won’t always use the camera.
It came with a camera so maybe that’s where the fine focus can be done but I won’t always use the camera.
What microscope was it? My guess is that there wouldn't be additional focus for the camera - it's mostly about keeping the stage exactly the right distance from the objective, but even with a fine focus knob, it can be tricky to feel like you've nailed it. Some images floating around of microscopic stuff have been focus stacked - to make up for the very limited focal plane depth, you can take several images at several heights and them compute them together to get effectively a larger depth in focus. Great for static samples and motorized microscopes, but not practical to do on a budget, and not really representative of what looking through the eyepiece at a sample will look like, even though they are excellent representations of what the thing actually looks like.
Aside from the accessory packages, IMO the best quality you can get for your dollar is usually on the used market. Takes some research, but a microscope surplussed from a clinic or university would probably get you considerably better optical quality, structure, and usability, though probably without a built in camera or a starter accessories pack (which you can get separately).
Aside from the accessory packages, IMO the best quality you can get for your dollar is usually on the used market. Takes some research, but a microscope surplussed from a clinic or university would probably get you considerably better optical quality, structure, and usability, though probably without a built in camera or a starter accessories pack (which you can get separately).
It’s one of the Amscope scopes. I got it based on what I read on R2R saying that’s all I need.
I agree with what you say about the used market and had looked there. Not sure why I didn’t ultimately go that route.
I agree with what you say about the used market and had looked there. Not sure why I didn’t ultimately go that route.
The scope feels solid for sure. Not sure why I can’t get a good view.
Similar threads
- Replies
- 9
- Views
- 493
- Replies
- 8
- Views
- 262
















