TDS is not affected by all natural elements as some do not dissolve. Neon and Helium for exampleThat is conductivity which can be correlated to TDS imperfectly and is affected by temperature. We use it because it is a simple test and works well enough for our purposes. True TDS which would be measured by evaporated solids weight is not affected by temperature.
TDS is the concentration of solids in the water. It is not affected by temperature any more than any other mass or weight measurement.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Reef Chemistry Puzzle #7
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
- Joined
- Dec 12, 2018
- Messages
- 311
- Reaction score
- 343
If oxygen why not flourine which is far more reactive than oxygen?
I'm probably wrong; Argon [Ar] is a natural element and it does not react with anything.
Mass as you mentioned is probably correct; it is not affected by temperature changes and every element has mass.
Huh?It has an inverse relationship to temperature.
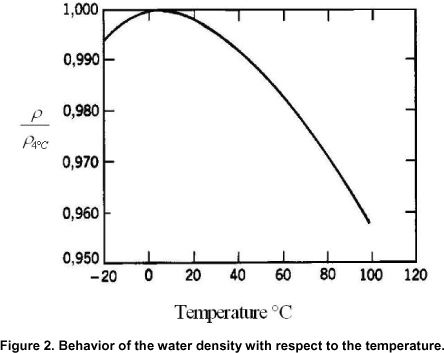
Read this quote by Randy
Let's start with some facts:
1. Salinity cannot ever change with temperature. Salinity is traditionally measured in weight of salt per weight of total water plus salt. Temperature cannot ever change that.
- Joined
- Jan 3, 2018
- Messages
- 850
- Reaction score
- 713
That is a good point, although even gases like Neon and Helium are absorbed to some degree in water and can theoretically affect the TDS even though they would not contribute as solids.TDS is not affected by all natural elements as some do not dissolve. Neon and Helium for example
I believe the issue is with measuring the salinity with a refractometer. If dried out and weighed of course temp doesn't effect it. But temp effects the measurement of salinity via refractometerHuh?
Read this quote by Randy
@Randy Holmes-Farley carbon? Binds with almost all elements though some more than others. Also never heard of carbon in liquid or gas form
I believe Neon and Helium are the only two truelly unreactive elements. But argon is almost completely no reactive.I'm probably wrong; Argon [Ar] is a natural element and it does not react with anything.
It would be the obvious one right? After all the time I spent thinking of obscure things it’s just mass.Mass as you mentioned is probably correct; it is not affected by temperature changes and every element has mass.
Temperature affects all salinity measurements, but it doesn’t affect the actual salinity in real life.I believe the issue is with measuring the salinity with a refractometer. If dried out and weighed of course temp doesn't effect it. But temp effects the measurement of salinity via refractometer
- Joined
- Dec 12, 2018
- Messages
- 311
- Reaction score
- 343
Temperature affects all salinity measurements, but it doesn’t affect the actual salinity in real life.
That is true as solutes remain the same they just move faster messing the electrodes on the TDS meter.
Every natural element has some impact on me, though some more than others.
But for it to be TDS it must be soluble in water; which not true for a lot of naturally occurring elements such as Helium.
I am going to go with a photon:
1. Every natural element has some impact on me, though some more than others.
Photons interact differently with different elements.
2. i am not changed in any way by a temperature increase.
I don't think a photon can be heated up because it is massless..(?)
1. Every natural element has some impact on me, though some more than others.
Photons interact differently with different elements.
2. i am not changed in any way by a temperature increase.
I don't think a photon can be heated up because it is massless..(?)
Yes, the energy of a photon is proportional to the temperature of an emitting body (for blackbody radiation), but an individual photon doesn't have a temperature.The average energy per photon is proportional to the temperature.
I was trying to key in on Randy's hint that all molecules are effected by a change in temperature, a photon (a non-molecule) made sense.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,059
- Reaction score
- 64,483
And the answer is...
1. Every natural element has some impact on me, though some more than others.
2. I am not changed in any way by a temperature increase.
What am I?
Seawater salinity! (and for this question, I'm defining that as including all nonwater components of seawater; see below)
Mass is almost a correct answer. Mass of seawater or mass of a reef tank would be a correct answer, because every element is present and adds to the mass of almost any reasonable amount of seawater. Mass is also not impacted by temperature. But "mass" alone without some qualifier of what mass we are talking about does not fit clue 1. Mass of the air in your home, for example, would not be impacted by all natural elements. thus, I would not give credit for the simple answer "mass".
Salinity has units of ppt, parts per thousand, such as mg/kg. It is a mass per mass unit. Thus, it fits clue #2 because we already determined mass is not impacted by temperature.
Does salinity fit clue #1? Harder...
Like many things in science, what seems like a simple definition on the surface really breaks down to uncertainty when you look deeper and deeper at it. Is salinity really just dissolved salts? Only dissolved salts? The general practical answer for seawater is that it doesn't matter since dissolved salts make up so much of the non water components in seawater that nothing else matters from a practical standpoint. But from a theoretical standpoint, does helium contribute to salinity? Why or why not? Just because it is not charged? What about boron. There is more boron as neutral B(OH)3 than as borate. Do we count it? If we dried out seawater, it would be there as weight. What about uncharged organics? Silica? Some authors seem to use seawater solids and salts interchangeably in the same paragraph, but that's obviously not the case.
Back to helium. Does it count in seawater salinity? You cannot dry out seawater and retain the helium, but it also turns out that you cannot fully dry seawater without losing some of the ions (which we presume certainly count). That is because some of the water binds strongly to the ions as they dry out, and that last bit of water is actually harder to removed than some of the ions that can shift to uncharged forms and escape to the air. Examples are HCl (from chloride and H+) and CO2 (from breakdown of carbonate).
There's no definition of seawater that would formally include helium since as a practical matter, it is never important, so no one bothers to consider it. If, as has been the formal case for the past 60 years, we take salinity to be defined as determined by conductivity, then uncharged molecules, from boric acid to silica to helium, do not contribute. This is a failing of that definition, though not one of any practical significance for actual seawater.
For purposes of this question, I'm choosing to define salinity as the mass of all dissolved nonwater components of seawater. That may not be anyone else's definition, but I think it makes great sense, and perhaps someday every chemical oceanographer will use this definition. lol
1. Every natural element has some impact on me, though some more than others.
2. I am not changed in any way by a temperature increase.
What am I?
Seawater salinity! (and for this question, I'm defining that as including all nonwater components of seawater; see below)
Mass is almost a correct answer. Mass of seawater or mass of a reef tank would be a correct answer, because every element is present and adds to the mass of almost any reasonable amount of seawater. Mass is also not impacted by temperature. But "mass" alone without some qualifier of what mass we are talking about does not fit clue 1. Mass of the air in your home, for example, would not be impacted by all natural elements. thus, I would not give credit for the simple answer "mass".
Salinity has units of ppt, parts per thousand, such as mg/kg. It is a mass per mass unit. Thus, it fits clue #2 because we already determined mass is not impacted by temperature.
Does salinity fit clue #1? Harder...
Like many things in science, what seems like a simple definition on the surface really breaks down to uncertainty when you look deeper and deeper at it. Is salinity really just dissolved salts? Only dissolved salts? The general practical answer for seawater is that it doesn't matter since dissolved salts make up so much of the non water components in seawater that nothing else matters from a practical standpoint. But from a theoretical standpoint, does helium contribute to salinity? Why or why not? Just because it is not charged? What about boron. There is more boron as neutral B(OH)3 than as borate. Do we count it? If we dried out seawater, it would be there as weight. What about uncharged organics? Silica? Some authors seem to use seawater solids and salts interchangeably in the same paragraph, but that's obviously not the case.
Back to helium. Does it count in seawater salinity? You cannot dry out seawater and retain the helium, but it also turns out that you cannot fully dry seawater without losing some of the ions (which we presume certainly count). That is because some of the water binds strongly to the ions as they dry out, and that last bit of water is actually harder to removed than some of the ions that can shift to uncharged forms and escape to the air. Examples are HCl (from chloride and H+) and CO2 (from breakdown of carbonate).
There's no definition of seawater that would formally include helium since as a practical matter, it is never important, so no one bothers to consider it. If, as has been the formal case for the past 60 years, we take salinity to be defined as determined by conductivity, then uncharged molecules, from boric acid to silica to helium, do not contribute. This is a failing of that definition, though not one of any practical significance for actual seawater.
For purposes of this question, I'm choosing to define salinity as the mass of all dissolved nonwater components of seawater. That may not be anyone else's definition, but I think it makes great sense, and perhaps someday every chemical oceanographer will use this definition. lol
.
Any Chem puzzle #8?And the answer is...
1. Every natural element has some impact on me, though some more than others.
2. I am not changed in any way by a temperature increase.
What am I?
Seawater salinity! (and for this question, I'm defining that as including all nonwater components of seawater; see below)
Mass is almost a correct answer. Mass of seawater or mass of a reef tank would be a correct answer, because every element is present and adds to the mass of almost any reasonable amount of seawater. Mass is also not impacted by temperature. But "mass" alone without some qualifier of what mass we are talking about does not fit clue 1. Mass of the air in your home, for example, would not be impacted by all natural elements. thus, I would not give credit for the simple answer "mass".
Salinity has units of ppt, parts per thousand, such as mg/kg. It is a mass per mass unit. Thus, it fits clue #2 because we already determined mass is not impacted by temperature.
Does salinity fit clue #1? Harder...
Like many things in science, what seems like a simple definition on the surface really breaks down to uncertainty when you look deeper and deeper at it. Is salinity really just dissolved salts? Only dissolved salts? The general practical answer for seawater is that it doesn't matter since dissolved salts make up so much of the non water components in seawater that nothing else matters from a practical standpoint. But from a theoretical standpoint, does helium contribute to salinity? Why or why not? Just because it is not charged? What about boron. There is more boron as neutral B(OH)3 than as borate. Do we count it? If we dried out seawater, it would be there as weight. What about uncharged organics? Silica? Some authors seem to use seawater solids and salts interchangeably in the same paragraph, but that's obviously not the case.
Back to helium. Does it count in seawater salinity? You cannot dry out seawater and retain the helium, but it also turns out that you cannot fully dry seawater without losing some of the ions (which we presume certainly count). That is because some of the water binds strongly to the ions as they dry out, and that last bit of water is actually harder to removed than some of the ions that can shift to uncharged forms and escape to the air. Examples are HCl (from chloride and H+) and CO2 (from breakdown of carbonate).
There's no definition of seawater that would formally include helium since as a practical matter, it is never important, so no one bothers to consider it. If, as has been the formal case for the past 60 years, we take salinity to be defined as determined by conductivity, then uncharged molecules, from boric acid to silica to helium, do not contribute. This is a failing of that definition, though not one of any practical significance for actual seawater.
For purposes of this question, I'm choosing to define salinity as the mass of all dissolved nonwater components of seawater. That may not be anyone else's definition, but I think it makes great sense, and perhaps someday every chemical oceanographer will use this definition. lol
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,059
- Reaction score
- 64,483
On the possible answer of photon, that's also complicated.
Photons clearly fit clue #2: not impacted by temperature.
But what about clue #1? Does every natural element impact photons?
Helium atims might or might not qualify. They will not absorb any visible light. But they might scatter it, especially if present as say, a pool of liquid helium. Photons might scatter off the surface, and maybe even from individual helium atoms.
I'd have to say that photon as an answer is as good as salinity as an answer, probably better, so I'll give credit for it.
Photons clearly fit clue #2: not impacted by temperature.
But what about clue #1? Does every natural element impact photons?
Helium atims might or might not qualify. They will not absorb any visible light. But they might scatter it, especially if present as say, a pool of liquid helium. Photons might scatter off the surface, and maybe even from individual helium atoms.
I'd have to say that photon as an answer is as good as salinity as an answer, probably better, so I'll give credit for it.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,059
- Reaction score
- 64,483
.
Any Chem puzzle #8?
Yes, after I think one up. lol
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,059
- Reaction score
- 64,483
TDS is not affected by all natural elements as some do not dissolve. Neon and Helium for example
Yes, I think TDS (which is always defined as measured by conductivity) fails because not all elements impact conductivity, and like mass as an answer, even if one did define it a different way, it needs a qualifier of what that is the TDS of. TDS of pure fresh water is not impacted by all elements,.
I still think mass is a better answer as all elements definitely affect a mass while, as you point out, it is questionable whether some elements affect salinity.And the answer is...
1. Every natural element has some impact on me, though some more than others.
2. I am not changed in any way by a temperature increase.
What am I?
Seawater salinity! (and for this question, I'm defining that as including all nonwater components of seawater; see below)
Mass is almost a correct answer. Mass of seawater or mass of a reef tank would be a correct answer, because every element is present and adds to the mass of almost any reasonable amount of seawater. Mass is also not impacted by temperature. But "mass" alone without some qualifier of what mass we are talking about does not fit clue 1. Mass of the air in your home, for example, would not be impacted by all natural elements. thus, I would not give credit for the simple answer "mass".
Salinity has units of ppt, parts per thousand, such as mg/kg. It is a mass per mass unit. Thus, it fits clue #2 because we already determined mass is not impacted by temperature.
Does salinity fit clue #1? Harder...
Like many things in science, what seems like a simple definition on the surface really breaks down to uncertainty when you look deeper and deeper at it. Is salinity really just dissolved salts? Only dissolved salts? The general practical answer for seawater is that it doesn't matter since dissolved salts make up so much of the non water components in seawater that nothing else matters from a practical standpoint. But from a theoretical standpoint, does helium contribute to salinity? Why or why not? Just because it is not charged? What about boron. There is more boron as neutral B(OH)3 than as borate. Do we count it? If we dried out seawater, it would be there as weight. What about uncharged organics? Silica? Some authors seem to use seawater solids and salts interchangeably in the same paragraph, but that's obviously not the case.
Back to helium. Does it count in seawater salinity? You cannot dry out seawater and retain the helium, but it also turns out that you cannot fully dry seawater without losing some of the ions (which we presume certainly count). That is because some of the water binds strongly to the ions as they dry out, and that last bit of water is actually harder to removed than some of the ions that can shift to uncharged forms and escape to the air. Examples are HCl (from chloride and H+) and CO2 (from breakdown of carbonate).
There's no definition of seawater that would formally include helium since as a practical matter, it is never important, so no one bothers to consider it. If, as has been the formal case for the past 60 years, we take salinity to be defined as determined by conductivity, then uncharged molecules, from boric acid to silica to helium, do not contribute. This is a failing of that definition, though not one of any practical significance for actual seawater.
For purposes of this question, I'm choosing to define salinity as the mass of all dissolved nonwater components of seawater. That may not be anyone else's definition, but I think it makes great sense, and perhaps someday every chemical oceanographer will use this definition. lol
But you’ve made your point well
Impatient much? Lol.
Any Chem puzzle #8?
Similar threads
- Replies
- 9
- Views
- 222
- Replies
- 16
- Views
- 426
- Replies
- 14
- Views
- 546
- Replies
- 15
- Views
- 406

















