- Joined
- Oct 7, 2017
- Messages
- 438
- Reaction score
- 388
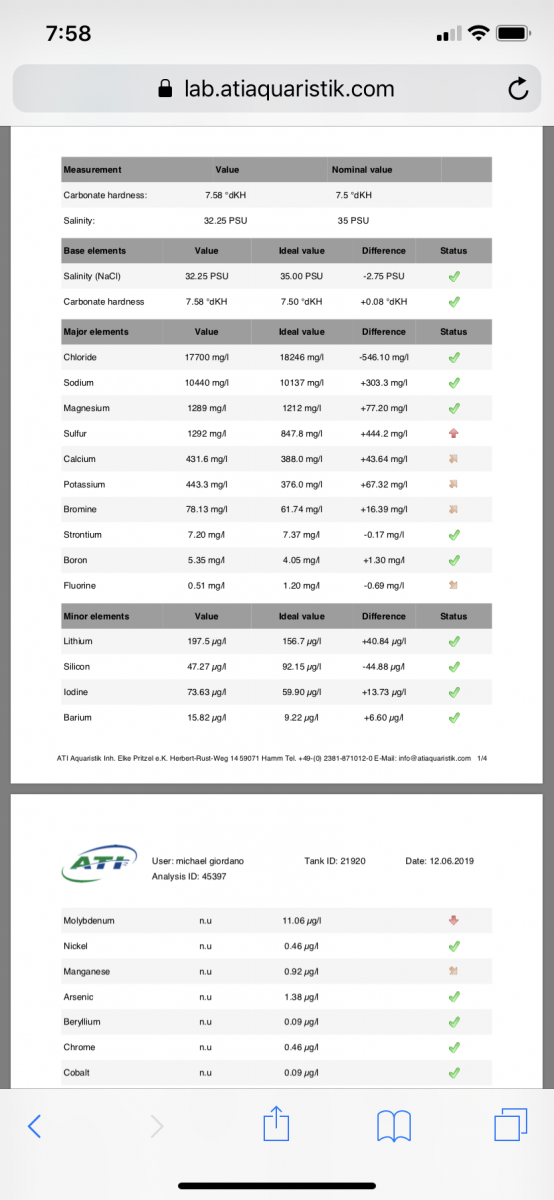
Got an ATI test completed and I have high sulfur and not sure why. My ro water sample says it has no sulfur in it. does anyone know if my level of sulfur is an issue for my reef? Really looking to maybe lower sulfur and if anyone has ideas how to lower it or is this bad level lol?