Let's be clear here. The energy of the photon has little to do with rates of photosynthesis. However, it does matter when it comes to effects on organic tissues.Led's by their nature have a high output of energy from a small area. It's only a matter of degrees. Blue to UV photons if course have high energy and concentrated (think lenses/magnifying glass) each can transfer enough energy to "burn" things as does any "color" with the right focus, think laser.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The use of UV light in LED
- Thread starter merereef
- Start date
- Tagged users None
Mastiffsrule
Where ever you go, there you are, so be nice 2 you
View BadgesExcellence Award
Reef Tank 365
Article Contributor
Reef Squad Emeritus
Hospitality Award
My Tank Thread
Led's by their nature have a high output of energy from a small area. It's only a matter of degrees. Blue to UV photons if course have high energy and concentrated (think lenses/magnifying glass) each can transfer enough energy to "burn" things as does any "color" with the right focus, think laser.
Thanks. Totals burned a nice armchair not paying attention.
- Joined
- Jun 17, 2016
- Messages
- 704
- Reaction score
- 769
Hi all,
I have a question about UV diodes on the HD52. I noticed the plastic bracing on top of my tank melted, and a chair I accidentally rested a lamp on that was still lit had burned a dot where the UV diode was located. Would that burning/melting be the case with violet, even though it is not a true UV range?
Just wondering?
This is interesting... someone ( a very well known reefer in the industry) mentioned that there is no need to run the uv and voilets above 50%. They make the fixture heat up more which i can confirm as ive checked.. the new red sea lights spectrum wise has lower uv and voilets.
- Joined
- Jun 17, 2016
- Messages
- 704
- Reaction score
- 769
So ive re ran the test same par with low uv and voilets and uv and voilets same percentage as the royal blue and blue LED. I matched the PAR. So they are the same. I found that with higher uv and voilets the corals started to grow green some of the acros and colours were better.. but with lower uv and voilets i still got good colour but the corals went from GREEN TO different colour. Also with lower uv and voilets the corals grew faster... this is with the same PAR. So i think there is something to this. Blues are run at 110 and voilets uv at 60%
- Joined
- Jun 17, 2016
- Messages
- 704
- Reaction score
- 769
- Joined
- Sep 18, 2017
- Messages
- 5,608
- Reaction score
- 3,451
That's a relatively cryptic response..Let's be clear here. The energy of the photon has little to do with rates of photosynthesis. However, it does matter when it comes to effects on organic tissues.
So what is the purpose of charts like this?
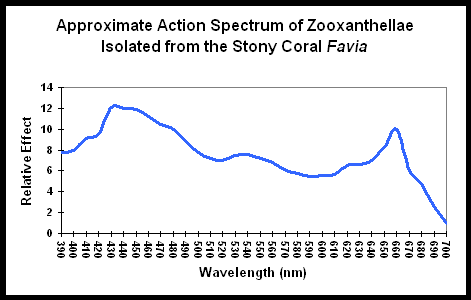
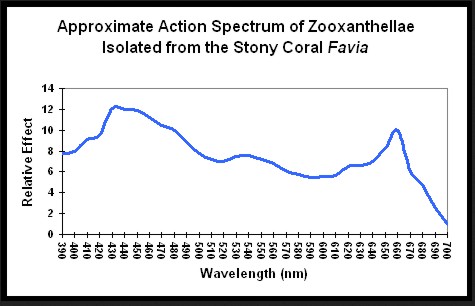
Lighting the Reef Aquarium – Spectrum or Intensity?
This month Dana and Miguel discuss the importance of spectrum and intensity of aquarium lighting.
Figure 1 demonstrates the light energy harvested by zooxanthellae isolated from the stony coral Favia, in a chart called an “action spectrum.” An action spectrum describes the relative effectiveness of energy at different wavelengths in producing particular biochemical or biological responses (such as oxygen evolution, carbon uptake, electron transport rate, etc., during photosynthesis).....
Maybe I'm missing a subtlety.
Granted it IS complicated..
The light field available for the zooxanthellae in the tissue differed strongly from the incident irradiance due to the combined action of multiple scattering and absorption, resulting in a wavelength-dependent local maximum of the spherically integrated quantum flux (i.e. scalar irradiance) in the upper tissue layers (Fig. 6).
Last edited:
- Joined
- Sep 18, 2017
- Messages
- 5,608
- Reaction score
- 3,451
Think you need to spread out the blame here a bit. Like this:Orphek is all that I know of. I do not guess to the ones with their proprietary diodes - no idea.
What most 405 and 415 is violet, not UV - EcoTech has lied about this since G2 or G3? ...I forget. They know perfectly well that these are not really UV.

Turbocharge your BLUE Stony Corals with 370nm UV Spotlights | Reef Builders | The Reef and Saltwater Aquarium Blog
The vast majority of reefers reading this definitely don't need a specialized spotlight for exotic ultraviolet spectrum – but since when is the reefing hobby one of necessity? However if you'
reefbuilders.com
That being said, best LED lights already do an awesome job of rendering all the colors of aquarium corals, and the UV in the 400 to 430nm range is probably ‘good enough’ for virtually all reefers. But if you wanted to push the envelope, dipping below 400nm would be a very interesting experiment.
Kind of a chicken or egg thing.
BTW Almost every "20000k" mh is/was no such thing either.
If one wants to assume "20000k-like" (whatever that means) 410nm certainly could be called "UV- like"...
There is no shortage of "market-speek".
Not defending it btw, BOTH are annoying.
Might as well throw in "actinic" to the mix as well.
. Relating to or denoting light able to cause photochemical reactions, as in photography, through having a significant short wavelength or ultraviolet component.
Pretty broad and fairly useless definition
Last edited:
The energy level of the photon matters not in photosynthesis (see Kirk's book Light and Photosynthesis in Aquatic Environments), what matters if the photon can be absorbed by the photopigment, which is what the action spectrum chart shows.That's a relatively cryptic response..
So what is the purpose of charts like this?

Lighting the Reef Aquarium – Spectrum or Intensity?
This month Dana and Miguel discuss the importance of spectrum and intensity of aquarium lighting.reefs.com
Maybe I'm missing a subtlety.
Granted it IS complicated..
- Joined
- Sep 18, 2017
- Messages
- 5,608
- Reaction score
- 3,451
I see your point but the bottom line in my orig question was, regardless of "where" it comes from there is a difference in quantum yield based on wavelength (wavelength = energy)..The energy level of the photon matters not in photosynthesis (see Kirk's book Light and Photosynthesis in Aquatic Environments), what matters if the photon can be absorbed by the photopigment, which is what the action spectrum chart shows.
It technically was "energy independent" though it would be hard to believe there is not some correlation.
i,e what causes the pigment absorption of a specific photon at a specific wavelength.
You have a reaction center that is err flexible yet selective and the energy level determines this to some degree?
Probably would need to go into the quantum mechanics probability type stuff.
Why is there even a gradient to the absorption curve?
But I digress..
The way I looked at it, is the PUR value of 400nm the same as the PUR value of say 450nm light?
Action spectra give an answer to that..regardless of the exact cause.
Secondary of course is the non-photosynthetic reactions.
INTRODUCTION
For well over half a century, it has been known that the energy conversion efficiency of incident photons to chemical energy by leaves is wavelength dependent (Hoover, 1937). This is due to several processes that can be divided into two classes. First, the absorption of incident irradiance by a leaf is wavelength dependent due to the different absorptance spectra of the different leaf pigments. Second, even on an absorbed light basis, different wavelengths have different quantum yields for CO2 fixation or O2 evolution: Red light (600 to 640 nm) has the highest quantum yield, whereas blue and green light (400 to 570 nm) are considerably less efficient in driving photosynthesis (McCree, 1972b; Inada, 1976; Evans, 1987). Maximum quantum yields for C3 leaves were found to be close to 0.093 mol CO2 fixed (Long et al., 1993) or 0.106 mol O2 evolved (Björkman and Demmig, 1987) per mol absorbed photons.
Three major causes for the wavelength dependence of the quantum yield for absorbed photons have been identified (i.e., absorption by photosynthetic carotenoids, absorption by nonphotosynthetic pigments, and an imbalanced excitation of the two photosystems) (Terashima et al., 2009). Photosynthetic carotenoids have absorption maxima for blue wavelengths and differ in their efficiency (35 to 90%) for excitation energy transfer to chlorophylls, depending on the type of carotenoid and its position within the photosynthetic apparatus, whereas the energy transfer efficiency in the antenna complexes from chlorophyll to chlorophyll is 100% (Croce et al., 2001; de Weerd et al., 2003a, 2003b; Caffarri et al., 2007). Nonphotosynthetic pigments, such as flavonoids and free carotenoids, also absorb light, predominantly in the UV region but also in the blue and green part of the spectrum (e.g., anthocyanins). Nonphotosynthetic pigments do not transfer any absorbed energy to the photosynthetic apparatus. Finally, the pigment composition and absorbance properties differ for photosystem I (PSI) and photosystem II (PSII); consequently, the balance of excitation between the two photosystems is wavelength dependent (Evans, 1986, 1987; Chow et al., 1990; Melis, 1991; Walters and Horton, 1995). Any imbalance in excitation of the two photosystems results in quantum yield losses (Pfannschmidt, 2005). However, a quantitative understanding of the relative contribution of each of these factors causing quantum yield losses is still lacking.

Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves
The quantum yield for CO[2] fixation is wavelength dependent due to (1) light absorption by nonphotosynthetic pigments, (2) inefficient energy transfer, and (3) the excitation balance between the two photosystems. The growth-light spectrum alters the ...
Photosynthetic carotenoids have absorption maxima for blue wavelengths and differ in their efficiency (35 to 90%) for excitation energy transfer to chlorophylls, depending on the type of carotenoid and its position within the photosynthetic apparatus, whereas the energy transfer efficiency in the antenna complexes from chlorophyll to chlorophyll is 100% (Croce et al., 2001; de Weerd et al., 2003a, 2003b; Caffarri et al., 2007).
Nonphotosynthetic pigments, such as flavonoids and free carotenoids, also absorb light, predominantly in the UV region but also in the blue and green part of the spectrum (e.g., anthocyanins). Nonphotosynthetic pigments do not transfer any absorbed energy to the photosynthetic apparatus.
Last edited:
Quantum yield could be described as the ratio of light absorbed and the end product (such as photosynthesis or fluorescence.) If 100 photons are absorbed and 100 are used in photosynthesis, then the yield is 1. If only 50 are used, then the yield is 0.5. In photosynthesis, the transfer of energy by peridinin to chl a and the reaction centers is very close to, if not 100%. Not necessarily so for other photopigments. It has to do with proximity of the photopigments (resonance energy transfer) but not the energy level of the wavelength.I see your point but the bottom line in my orig question was, regardless of "where" it comes from there is a difference in quantum yield based on wavelength (wavelength = energy)..
It technically was "energy independent" though it would be hard to believe there is not some correlation.
i,e what causes the pigment absorption of a specific photon at a specific wavelength.
You have a reaction center that is err flexible yet selective and the energy level determines this to some degree?
Probably would need to go into the quantum mechanics probability type stuff.
Why is there even a gradient to the absorption curve?
But I digress..
The way I looked at it, is the PUR value of 400nm the same as the PUR value of say 450nm light?
Action spectra give an answer to that..regardless of the exact cause.
Secondary of course is the non-photosynthetic reactions.

Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves
The quantum yield for CO[2] fixation is wavelength dependent due to (1) light absorption by nonphotosynthetic pigments, (2) inefficient energy transfer, and (3) the excitation balance between the two photosystems. The growth-light spectrum alters the ...www.ncbi.nlm.nih.gov
- Joined
- Jun 17, 2016
- Messages
- 704
- Reaction score
- 769
Quantum yield could be described as the ratio of light absorbed and the end product (such as photosynthesis or fluorescence.) If 100 photons are absorbed and 100 are used in photosynthesis, then the yield is 1. If only 50 are used, then the yield is 0.5. In photosynthesis, the transfer of energy by peridinin to chl a and the reaction centers is very close to, if not 100%. Not necessarily so for other photopigments. It has to do with proximity of the photopigments (resonance energy transfer) but not the energy level of the wavelength.
Have you ever tested what spectrum mix or what NM LED creates the highest PUR? The new orphek LED BARS create 100PUR ACCORDING TO THE seneye tester... and red sea claim there LED creates 95% pur. Was wondering what we can change on our LED to give the highest possible PUR.
From what I've seen, the violet, blue, and red LEDs produce the most PUR. Which Orphek light bars are you referring to? Blue Plus, etc.?Have you ever tested what spectrum mix or what NM LED creates the highest PUR? The new orphek LED BARS create 100PUR ACCORDING TO THE seneye tester... and red sea claim there LED creates 95% pur. Was wondering what we can change on our LED to give the highest possible PUR.
- Joined
- Jun 17, 2016
- Messages
- 704
- Reaction score
- 769
From what I've seen, the violet, blue, and red LEDs produce the most PUR. Which Orphek light bars are you referring to? Blue Plus, etc.?
Yes the orphek light bar im referring to is blue plus think its called blue sky, ive just had a look on their website and that bar only utilises royal blue and cyan
Last edited:
I have an Orphek Blue Plus strip. Peaks are at 420nm (violet), 450nm (violet/blue), and 490nm (cyan.)Yes the orphek light bar im referring to is blue plus think its called blue sky, ive just had a look on their website and that bar only utilises royal blue and cyan
- Joined
- Sep 18, 2017
- Messages
- 5,608
- Reaction score
- 3,451
Orphek seems to change things by the minute..I have an Orphek Blue Plus strip. Peaks are at 420nm (violet), 450nm (violet/blue), and 490nm (cyan.)
Lists 8 different diodes
See 0:36
Orphek seems to change things by the minute..
Lists 8 different diodes
See 0:36
The Blue Plus appears to have the same diodes as the one I have.
- Joined
- Sep 18, 2017
- Messages
- 5,608
- Reaction score
- 3,451
The Blue Plus appears to have the same diodes as the one I have.
to is blue plus think its called blue sky
That is my initial mistake. Was thinking about the blue sky not blue plus. 2 very different lights
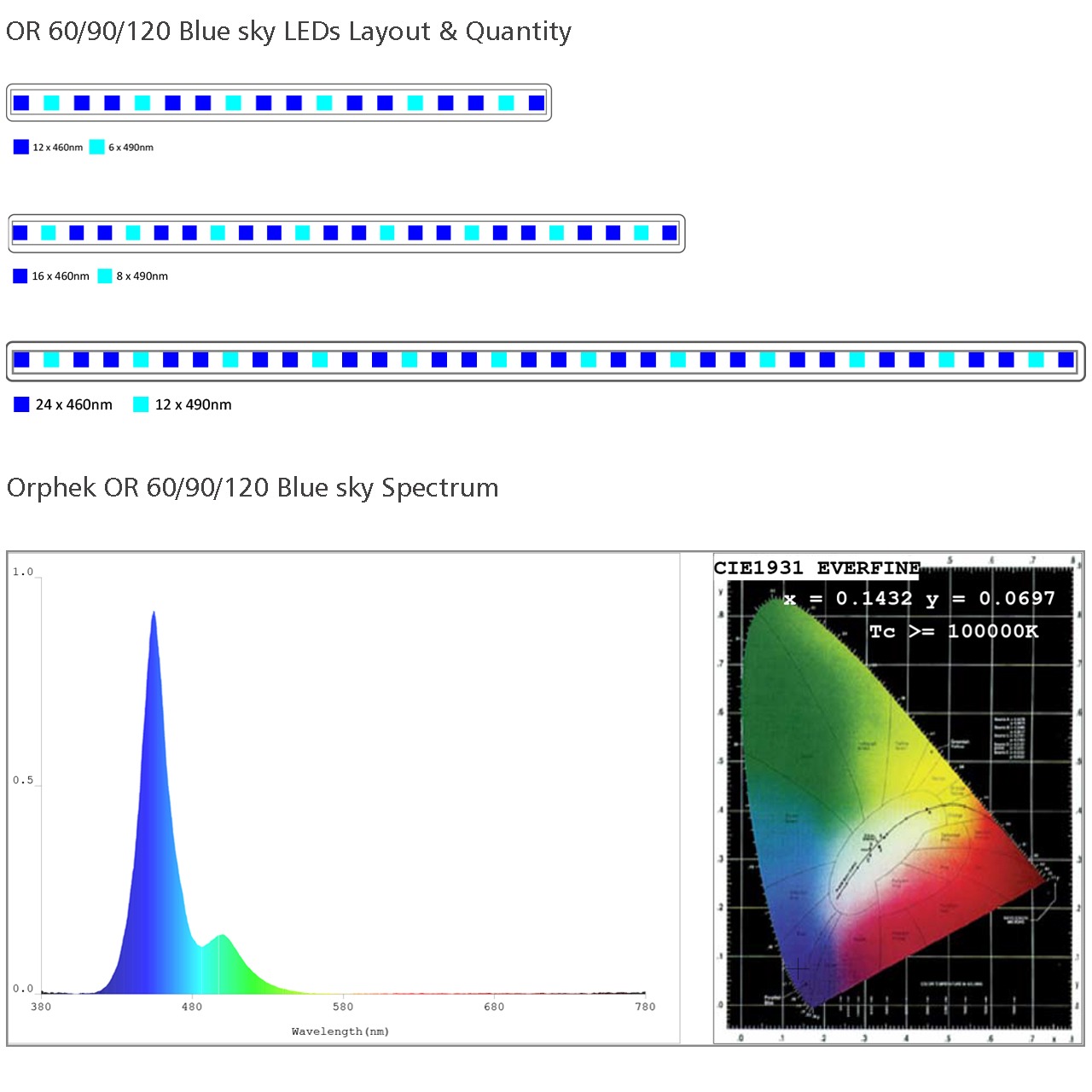
Yea got off track. Orig series had a blue sky w/ just 460 490 diodes.
Now they have the blue plus.. and no blue sky w 8 diode spectrums.
or
???????????
On a quick glance looks like they don't keep the color ratios as models get bigger.
There are no 460's is the 120..
I've got about a dozen Orphek LED strip lights. I'll see what I've got and test when I get a chance.That is my initial mistake. Was thinking about the blue sky not blue plus. 2 very different lights

Yea got off track. Orig series had a blue sky w/ just 460 490 diodes.
Now they have the blue plus.. and no blue sky w 8 diode spectrums.
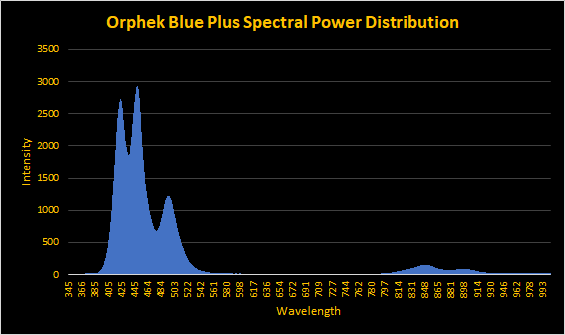
or

???????????
On a quick glance looks like they don't keep the color ratios as models get bigger.
Definitely the highest PUR I've seen as measured with a Seneye device. 'White' LEDs are in the 30's while cooler ones can be as high as ~90%. If it's really 100%, what does that say about the intensity (PAR) we should maintain?
Similar threads
- Replies
- 9
- Views
- 311
- Replies
- 16
- Views
- 259
- Replies
- 1
- Views
- 67






















