maroun.c
Moderator
View Badges
Staff member
Super Moderator
Excellence Award
Reef Of The Month
Photo of the Month
Article Contributor
My Tank Thread
Appreciate your input about below Triton results, What to worry about and what not.
Also any input about my correction plan
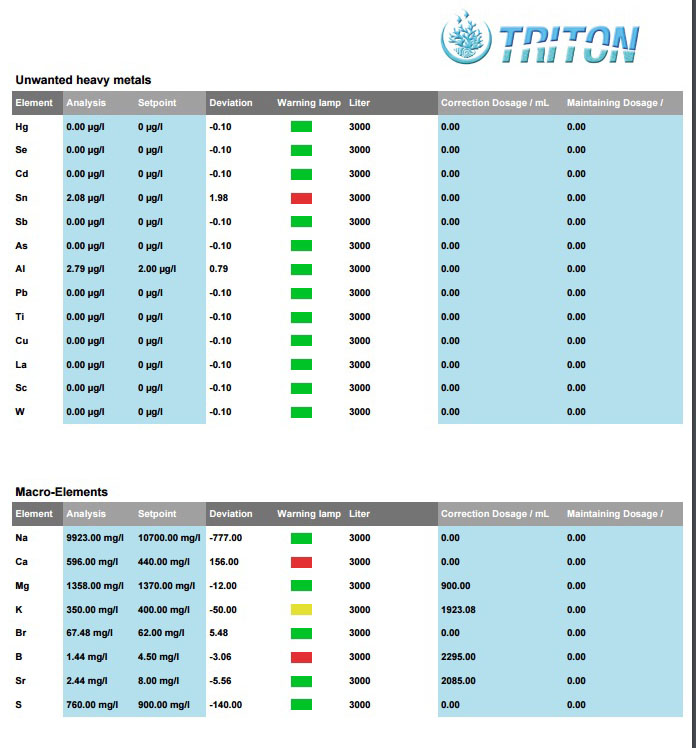
SN: Tin not Strontium right? Any idea what caused it and how to deal with it?
Will a heavy metal resin like Cuprisorb take it out? any advice what to watch out for?
Ca: is a mishap and inline with my tests so will correct.
B: Boron? is it important? Anyneed to adjust and how?
SR: Strontium at 1/4 of set point why not a yellow or red indicator?
S: Sulfur at 140 less than set point? need to correct? how
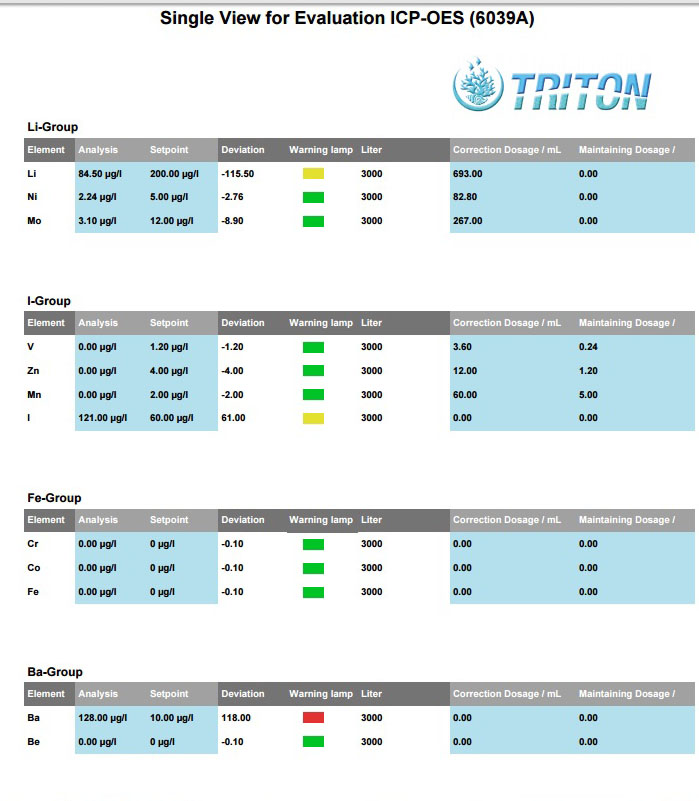
Ba Barium at 128 instead of setpoint 118 and flagged red is this important?any idea what caused it?
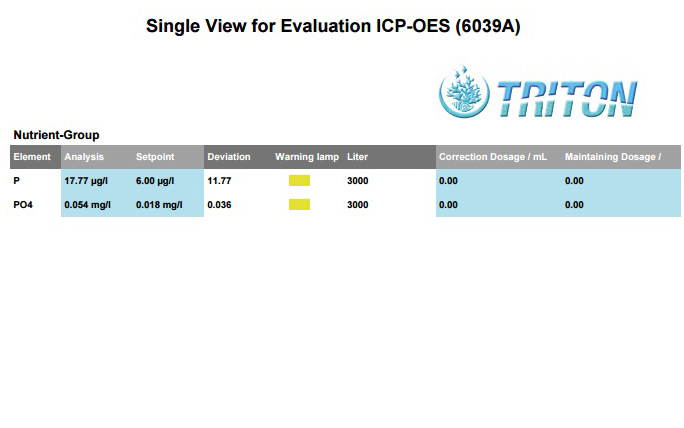

 Thanks for any help or suggestions.
Thanks for any help or suggestions.
Also any input about my correction plan
SN: Tin not Strontium right? Any idea what caused it and how to deal with it?
Will a heavy metal resin like Cuprisorb take it out? any advice what to watch out for?
Ca: is a mishap and inline with my tests so will correct.
B: Boron? is it important? Anyneed to adjust and how?
SR: Strontium at 1/4 of set point why not a yellow or red indicator?
S: Sulfur at 140 less than set point? need to correct? how
Ba Barium at 128 instead of setpoint 118 and flagged red is this important?any idea what caused it?














