I have been running my Reef Angel controller for about two weeks now with a PH probe. I have noticed that the PH typically is from 8.04 - 7.85. See chart
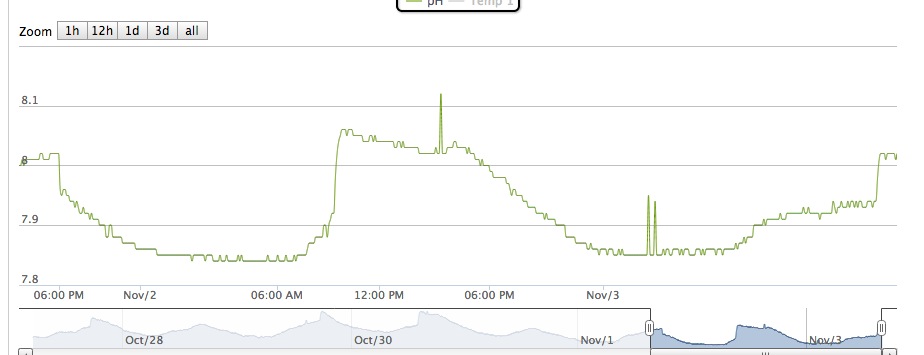
The PH seems on the low side of what I would like it to be (it is about the same on an Salifert and API test kit), but was wondering if this is a typical drop in PH ~ .15.
Params:
dKH - 8-9
Calc - 450-500
Sump:
Refugium with Chaeto and Dragons Breadth (24hr light schedule)
GFO and Carbon in BRS Two Chamber reactor (24x7)
Tank
Mostly softies and LPS, a handful of SPS frags (all seem to be ok, except my Blue Tunius - but think that is a light issue)
I have read of some ways to increase and maintain PH, but have not decided on what my course of action will be:
Kalk - I do not have ATO setup yet and am scared that my controller will malfunction and overdose tank - so i would rather not go this method
C02 - I have not done the outside airstone test yet. My tank sits next to outside wall so I can drill air tube to fresh air, but am afraid that pest control will kill my tank when they spray insectided around perminter of the house (plus will give ants a nice path inside - they seem to be drawn to water)
MacroAlgaee - I have Chaeto (not growing well) and dragons Breadth - on 24x7 - any other varieties I should get that would do better?
Chemical - I was thinking about using Aquavitro Balance to drive PH up without effecting Alk/Calc and still do my two-part - or just dosing more two-part to get my alk higher
The PH seems on the low side of what I would like it to be (it is about the same on an Salifert and API test kit), but was wondering if this is a typical drop in PH ~ .15.
Params:
dKH - 8-9
Calc - 450-500
Sump:
Refugium with Chaeto and Dragons Breadth (24hr light schedule)
GFO and Carbon in BRS Two Chamber reactor (24x7)
Tank
Mostly softies and LPS, a handful of SPS frags (all seem to be ok, except my Blue Tunius - but think that is a light issue)
I have read of some ways to increase and maintain PH, but have not decided on what my course of action will be:
Kalk - I do not have ATO setup yet and am scared that my controller will malfunction and overdose tank - so i would rather not go this method
C02 - I have not done the outside airstone test yet. My tank sits next to outside wall so I can drill air tube to fresh air, but am afraid that pest control will kill my tank when they spray insectided around perminter of the house (plus will give ants a nice path inside - they seem to be drawn to water)
MacroAlgaee - I have Chaeto (not growing well) and dragons Breadth - on 24x7 - any other varieties I should get that would do better?
Chemical - I was thinking about using Aquavitro Balance to drive PH up without effecting Alk/Calc and still do my two-part - or just dosing more two-part to get my alk higher

















