- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
Dear All,
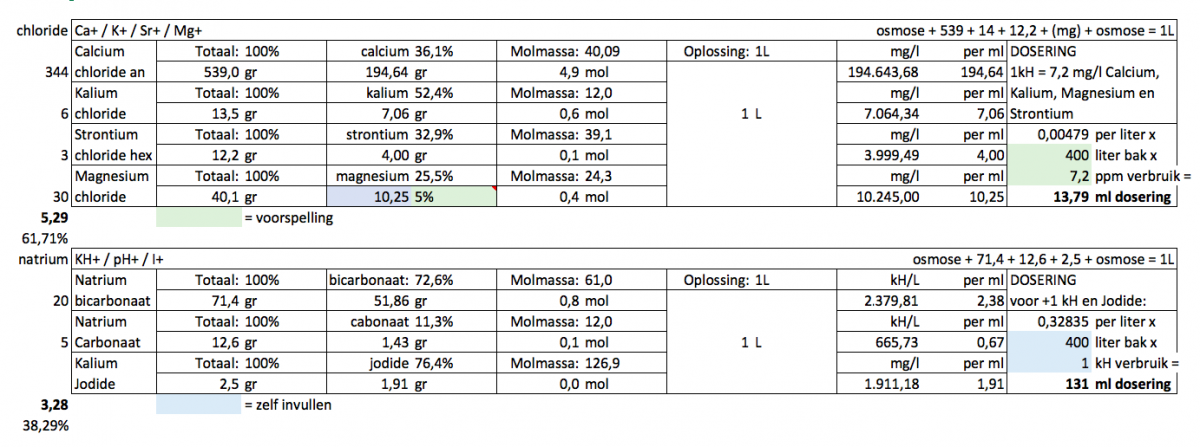
I wondered if anyone could give me some feedback on my version of 2-part. Sorry for the Dutch but I guess you understand what it means. Bluefield I fill in, green is expected outcome.
Potassium: based on randy's experience with Dow's
Strontium:Calcium, 1:50, this ratio i got from balling
Magnesium: I choose 5% for magnesium&calcium combined (as some Mg is used instead of Ca regarding the 7,2 to 1kH usage), I will still do a waterchange with salt that is very high in Mg (like 1600)
Iodine: dosage based on Jimmy54 advice
kH 85% sodiumbicarbonate and 15% soda ash to make 84g solution (counter the pH drop a bit).
I am also working on making stock solution for magnesium & Iron. still trying to figure out how much EDDHA & DTPA chelate to use for iron.. (or maybe there is a better chelate?). I do not know if is will remain stable if I dissolve some in 1L reverse osmoses., mono-natriumcitraat with a drop of vodka I will use for the manganese solution (not for Fe else it will release iron too quick and bond with phosphate and settle.. i do not intent to reduce phosphate, just use iron as a trace element, therefore I need a different chelate). Maybe someone has a recipe for me?
Please if anyone could give me some feedback, please let me know what you think.
I wondered if anyone could give me some feedback on my version of 2-part. Sorry for the Dutch but I guess you understand what it means. Bluefield I fill in, green is expected outcome.
Potassium: based on randy's experience with Dow's
Strontium:Calcium, 1:50, this ratio i got from balling
Magnesium: I choose 5% for magnesium&calcium combined (as some Mg is used instead of Ca regarding the 7,2 to 1kH usage), I will still do a waterchange with salt that is very high in Mg (like 1600)
Iodine: dosage based on Jimmy54 advice
kH 85% sodiumbicarbonate and 15% soda ash to make 84g solution (counter the pH drop a bit).
I am also working on making stock solution for magnesium & Iron. still trying to figure out how much EDDHA & DTPA chelate to use for iron.. (or maybe there is a better chelate?). I do not know if is will remain stable if I dissolve some in 1L reverse osmoses., mono-natriumcitraat with a drop of vodka I will use for the manganese solution (not for Fe else it will release iron too quick and bond with phosphate and settle.. i do not intent to reduce phosphate, just use iron as a trace element, therefore I need a different chelate). Maybe someone has a recipe for me?
Please if anyone could give me some feedback, please let me know what you think.
















