Approximating and Maintaining Water Chemistry of a Meromictic Lake
I'm starting a new project as a continuation of my Stromatolite Reef setup maintained with hypersaline (85ppt) water and 2-part calcium + alkalinity dosing for biologically-mediated precipitation of CaCO3 and stromatolite development.
The next tank I'm planning will attempt to model the fascinating system of Green Lake, New York. a small, but very deep lake near Syracuse. Green Lake is a meromictic lake, with its distinct and permanent water level stratification maintained by its depth, steep sides and the influx of saline water via deep underwater springs.
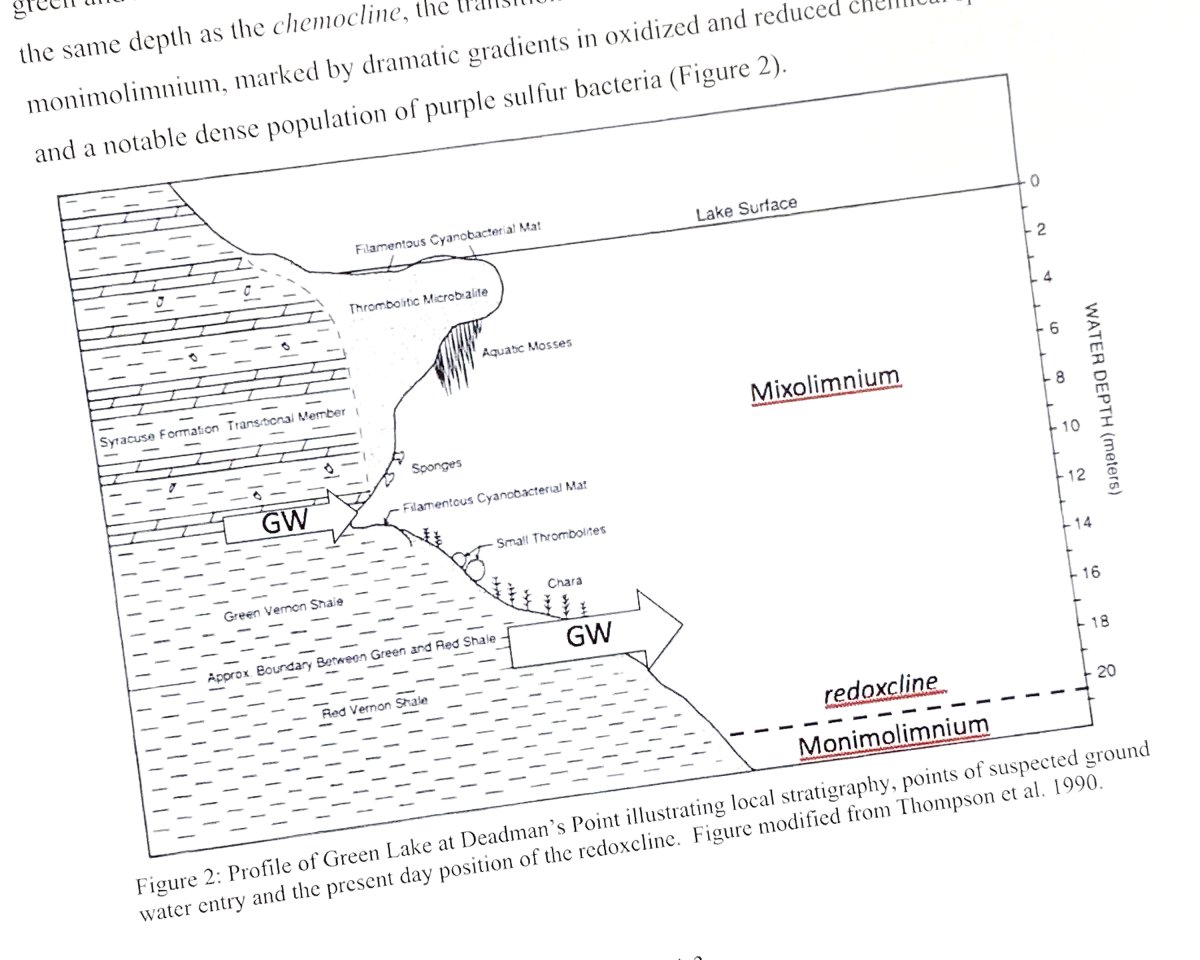
(Source: Brunskill, G. J. Ludlam, S. D., (1969), FAYETTEVILLE GREEN LAKE, NEW YORK. I. PHYSICAL AND CHEMICAL LIMNOLOGY, Limnology and Oceanography, 14, doi: 10.4319/lo.1969.14.6.0817.)
Among other fascinating features of Green Lake is the thrombolite reef structure at Dead Man's Point on the lake's eastern shore. I intend to model this an a living aquarium aquascape.

(Creative Commons image: https://en.wikipedia.org/wiki/Green_Lake_(New_York)#/media/File:GreenLakesDeadmanPoint.jpg)
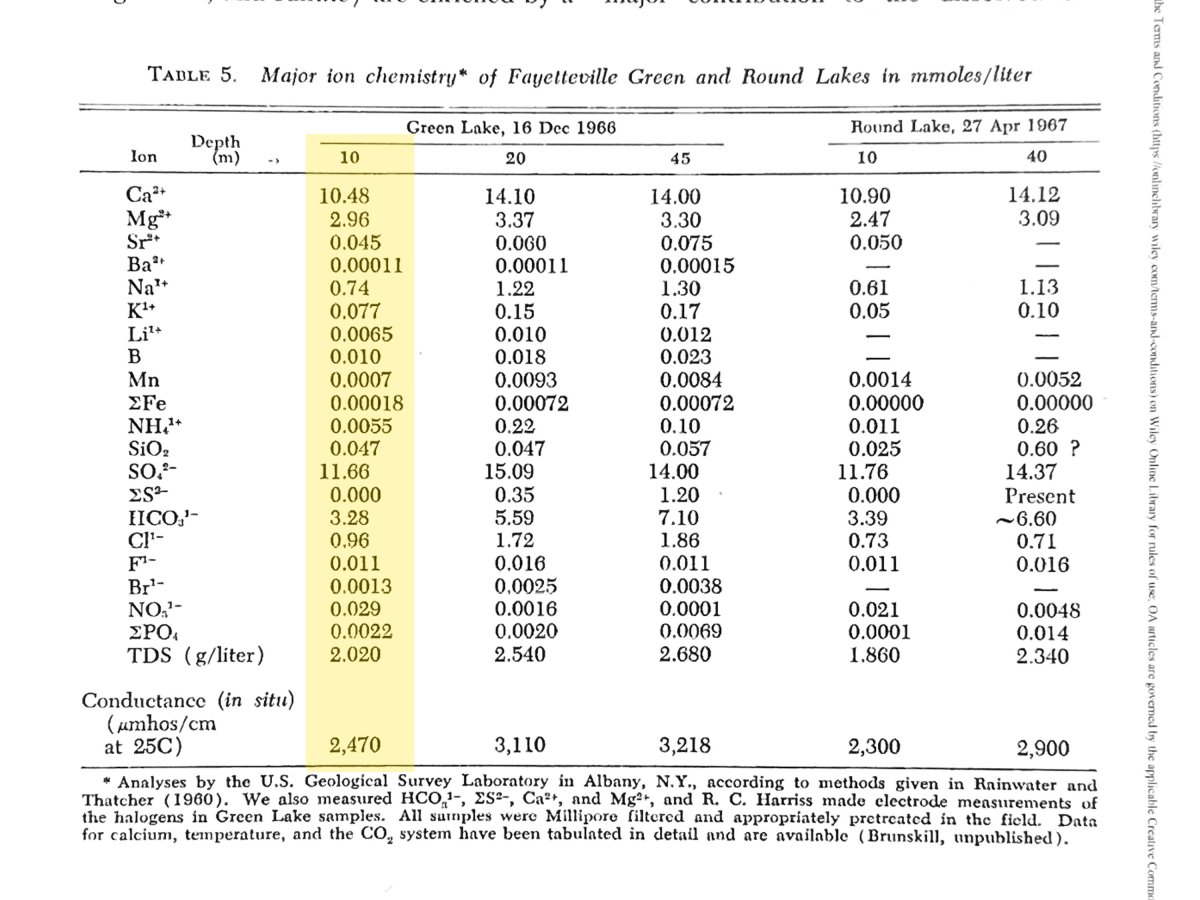
I'm no chemist, so I could use some help with a starting point for approximating Green Lake's water chemistry, especially the high calcium and bicarbonate concentration that supports the microbial thrombolite development. Here's a table from the Brunskill and Ludlam paper (also shown above) with ion concentrations as measured almost fifty years ago. I'm most interested in conditions at shallower depths and above the level of the chemocline, so see the table below for the values highlighted in yellow. Apparently the lake ecosystem has been maintained in a more or less healthy and stable condition in recent years, so these would not be expected to have changed much.
(Source: Brunskill, G. J. Ludlam, S. D., (1969), FAYETTEVILLE GREEN LAKE, NEW YORK. I. PHYSICAL AND CHEMICAL LIMNOLOGY, Limnology and Oceanography, 14, doi: 10.4319/lo.1969.14.6.0817.)
Green Lake is surprisingly salty for an inland lake. Groundwater flux from the Silurian marine shales, dolomites and other rock layers account for the elevated sulfate, sodium and chloride levels.
Do any readers here have tips or ideas for approximating and maintaining this chemistry? If I can get the organisms to grow, they will deplete calcium and alkalinty, so I also need a plan for replenishment. The aquarium I have in mind os just ten gallons, so a 2-part dosing regime should be acceptable for maintenence. Could an off-the-shelf 2-part be acceptable, or will I have to mix up my own salts?
I think that trace mineral concentrations are less critical. I don't intend to dose for those and I should be able to maintain growth of the Cyanobacteria and associated thrombolite organisms with occasional guesstimated dosing of BG-11 or other nutrient medium solution.
Thanks for reading. Like I mentioned I do't have a lot of practice with water chemistry, so any hints, shortcuts or explanations would be greatly appreciated.
I'm starting a new project as a continuation of my Stromatolite Reef setup maintained with hypersaline (85ppt) water and 2-part calcium + alkalinity dosing for biologically-mediated precipitation of CaCO3 and stromatolite development.
The next tank I'm planning will attempt to model the fascinating system of Green Lake, New York. a small, but very deep lake near Syracuse. Green Lake is a meromictic lake, with its distinct and permanent water level stratification maintained by its depth, steep sides and the influx of saline water via deep underwater springs.
(Source: Brunskill, G. J. Ludlam, S. D., (1969), FAYETTEVILLE GREEN LAKE, NEW YORK. I. PHYSICAL AND CHEMICAL LIMNOLOGY, Limnology and Oceanography, 14, doi: 10.4319/lo.1969.14.6.0817.)
Among other fascinating features of Green Lake is the thrombolite reef structure at Dead Man's Point on the lake's eastern shore. I intend to model this an a living aquarium aquascape.
(Creative Commons image: https://en.wikipedia.org/wiki/Green_Lake_(New_York)#/media/File:GreenLakesDeadmanPoint.jpg)
I'm no chemist, so I could use some help with a starting point for approximating Green Lake's water chemistry, especially the high calcium and bicarbonate concentration that supports the microbial thrombolite development. Here's a table from the Brunskill and Ludlam paper (also shown above) with ion concentrations as measured almost fifty years ago. I'm most interested in conditions at shallower depths and above the level of the chemocline, so see the table below for the values highlighted in yellow. Apparently the lake ecosystem has been maintained in a more or less healthy and stable condition in recent years, so these would not be expected to have changed much.
(Source: Brunskill, G. J. Ludlam, S. D., (1969), FAYETTEVILLE GREEN LAKE, NEW YORK. I. PHYSICAL AND CHEMICAL LIMNOLOGY, Limnology and Oceanography, 14, doi: 10.4319/lo.1969.14.6.0817.)
Green Lake is surprisingly salty for an inland lake. Groundwater flux from the Silurian marine shales, dolomites and other rock layers account for the elevated sulfate, sodium and chloride levels.
Do any readers here have tips or ideas for approximating and maintaining this chemistry? If I can get the organisms to grow, they will deplete calcium and alkalinty, so I also need a plan for replenishment. The aquarium I have in mind os just ten gallons, so a 2-part dosing regime should be acceptable for maintenence. Could an off-the-shelf 2-part be acceptable, or will I have to mix up my own salts?
I think that trace mineral concentrations are less critical. I don't intend to dose for those and I should be able to maintain growth of the Cyanobacteria and associated thrombolite organisms with occasional guesstimated dosing of BG-11 or other nutrient medium solution.
Thanks for reading. Like I mentioned I do't have a lot of practice with water chemistry, so any hints, shortcuts or explanations would be greatly appreciated.
Last edited:




















