Isn't calcium carbonate powder the basis for coral snow?How can I use this product to lower phosphates in my aquarium? Would I need a fine filter sock (1 micron?) to export the phosphate-saturated calcium carbonate?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Aragonite Sand And Phosphate Adsorption
- Thread starter Dan_P
- Start date
- Tagged users None
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
How can I use this product to lower phosphates in my aquarium? Would I need a fine filter sock (1 micron?) to export the phosphate-saturated calcium carbonate?
How much would you have to add to the aquarium? A TSP per 0.25 L?
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
I was wondering the same thing. Calcium carbonate is used in brewing. I wonder if a home brewer who had a reef aquarium started it allIsn't calcium carbonate powder the basis for coral snow?
Probably right. I can see a lot of cross over that might be applied to both hobbies and not a bad way to enjoy the tankI was wondering the same thing. Calcium carbonate is used in brewing. I wonder if a home brewer who had a reef aquarium started it all
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
Probably right. I can see a lot of cross over that might be applied to both hobbies and not a bad way to enjoy the tank
Ah, so it’s probably not going to be cost effective in a very large tank.How much would you have to add to the aquarium? A TSP per 0.25 L?
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
Aragonite Sand And Phosphate Adsorption. Adsorption Kinetics
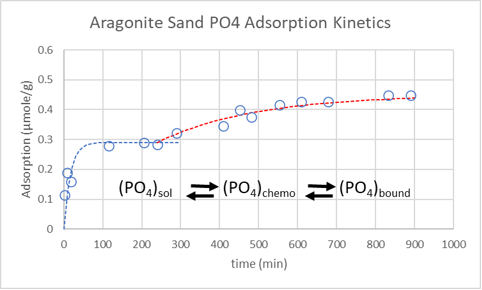
In this study, I used strong, mechanical mixing to suspend 20 g of washed new aragonite sugar sand in 1 L of Instant Ocean with 2.2 ppm phosphate. The adsorption of phosphate is rapid followed by a slower adsorption as reported by Millero. The lag time fir the slower addition rate was not observed for aragonite and might be an effect of a larger particle size used in this experiment. Repeated desorption-adsorption cycles slowly blend the two kinetic events into one where there is no longer a lag time.
Coupling this observation with the desorption data just posted, I am investigating phosphate adsorption by sand with the working hypothesis that the rapid adsorption represents a fast chemisorption followed by a slower reaction that binds phosphate to the surface. This bound phosphate might not desorb. I am also thinking that the slow adsorption kinetics represents the conversion rate of chemosorbed phosphate to bound phosphate with the subsequent freeing up of a chemisorption site and immediate chemisorption of phosphate.
Happy New Year!
In this study, I used strong, mechanical mixing to suspend 20 g of washed new aragonite sugar sand in 1 L of Instant Ocean with 2.2 ppm phosphate. The adsorption of phosphate is rapid followed by a slower adsorption as reported by Millero. The lag time fir the slower addition rate was not observed for aragonite and might be an effect of a larger particle size used in this experiment. Repeated desorption-adsorption cycles slowly blend the two kinetic events into one where there is no longer a lag time.
Coupling this observation with the desorption data just posted, I am investigating phosphate adsorption by sand with the working hypothesis that the rapid adsorption represents a fast chemisorption followed by a slower reaction that binds phosphate to the surface. This bound phosphate might not desorb. I am also thinking that the slow adsorption kinetics represents the conversion rate of chemosorbed phosphate to bound phosphate with the subsequent freeing up of a chemisorption site and immediate chemisorption of phosphate.
Happy New Year!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,474
- Reaction score
- 63,874
Aragonite Sand And Phosphate Adsorption. Adsorption Kinetics
In this study, I used strong, mechanical mixing to suspend 20 g of washed new aragonite sugar sand in 1 L of Instant Ocean with 2.2 ppm phosphate. The adsorption of phosphate is rapid followed by a slower adsorption as reported by Millero. The lag time fir the slower addition rate was not observed for aragonite and might be an effect of a larger particle size used in this experiment. Repeated desorption-adsorption cycles slowly blend the two kinetic events into one where there is no longer a lag time.
Coupling this observation with the desorption data just posted, I am investigating phosphate adsorption by sand with the working hypothesis that the rapid adsorption represents a fast chemisorption followed by a slower reaction that binds phosphate to the surface. This bound phosphate might not desorb. I am also thinking that the slow adsorption kinetics represents the conversion rate of chemosorbed phosphate to bound phosphate with the subsequent freeing up of a chemisorption site and immediate chemisorption of phosphate.
Happy New Year!

I agree that there may be changes in the surface structure, and that may be why some of these things happen slowly.
Where or from what vendor did the calcium reactor chunks come from? Did they look like mined aragonite or something removed from the ocean bottom? I am interested because the CaribSea Rubble that I have does nor seem to be adsorbing much phosphate. By the way, it looks like mined aragonite because of the occasional crystal found in some chunks.
I used Carib Sea ARM large chunks. They were pure aragonite chunks from the ocean. They have changed. The ARM now is mined calcite, unless it is changed again.
Rubble was never good as CaRx media because of too many shells in various forms and not enough real/exposed aragonite that can dissolve - the coating that the various life forms left makes any aragonite in them hard to access. I am not surprised that you are struggling with it. If it was not sold as reactor media, then it probably is not pure aragonite.
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
I agree that there may be changes in the surface structure, and that may be why some of these things happen slowly.
Subsequent experiments continue to show that more phosphate binds than desorbs, even after many desorption steps with Instant Ocean when. phosphate desorption becomes undetectable. This makes me wonder whether phosphate adsorption by aragonite surfaces that we have in our aquarium is not adequately described by Millero’s results. Also, @jda huge adsorption results not agreeing with Millero’s observations might now be understood by “irreversible” binding of phosphate though I haven’t been able to reproduce the high rate yet.
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
I went ahead and bought CaribSea ARM coarse medium. not knowing how close it came to the material used 4 years ago. I will give it a try anyway and we can go from go from there, possibly identifying a source of a purer form of aragonite.I used Carib Sea ARM large chunks. They were pure aragonite chunks from the ocean. They have changed. The ARM now is mined calcite, unless it is changed again.
Rubble was never good as CaRx media because of too many shells in various forms and not enough real/exposed aragonite that can dissolve - the coating that the various life forms left makes any aragonite in them hard to access. I am not surprised that you are struggling with it. If it was not sold as reactor media, then it probably is not pure aragonite.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,474
- Reaction score
- 63,874
Here’s an idea. Track alkalinity during the tests.
Fresh aragonite surfaces are good seed crystals for more precipitation of calcium carbonate, especially in raw salt water.
Any precipitating calcium carbonate can incorporate and bury some calcium phosphate, that cannot then desorb.
Fresh aragonite surfaces are good seed crystals for more precipitation of calcium carbonate, especially in raw salt water.
Any precipitating calcium carbonate can incorporate and bury some calcium phosphate, that cannot then desorb.
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
I looked at alkalinity in a stirred Instant Ocean-aragonite sand mixture as a function of time. Alkalinity was rock solid. But…I am starting a series of absorption-desorption experiments tomorrow and will add the alkalinity study.Here’s an idea. Track alkalinity during the tests.
Fresh aragonite surfaces are good seed crystals for more precipitation of calcium carbonate, especially in raw salt water.
Any precipitating calcium carbonate can incorporate and bury some calcium phosphate, that cannot then desorb.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,474
- Reaction score
- 63,874
I looked at alkalinity in a stirred Instant Ocean-aragonite sand mixture as a function of time. Alkalinity was rock solid. But…I am starting a series of absorption-desorption experiments tomorrow and will add the alkalinity study.
If you can, monitor pH too. When I added aragonite sand to my tank years ago, I couldn’t see any alk change, but pH dropped a bit right away and that may be a more sensitive way to detect loss of carbonate.
The Alk in my long term test has gone from 8.9ish to 6.7ish (23ppm phos, 2 kgs sand, 6 litres water). May be relevant that when I was adding a bag of sand last year, immediately downstream of my kalkwasser, the kalk didn’t have the same oompf, with regard to pH, which is why I moved the kalk dosing line.If you can, monitor pH too. When I added aragonite sand to my tank years ago, I couldn’t see any alk change, but pH dropped a bit right away and that may be a more sensitive way to detect loss of carbonate.
Did this again, same process but have waited 96hrs to test, results show slightly over 0.03 on salient, certainly lower than 0.1ppm.I discarded the water from this sample and replaced it with 0 phos 20mls new salt water, waited 48 hrs with the occasional swirl. Tested at near 0.25 ppm ish, certainly higher than 0.1 on salifert.
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
Will add the pH observation to the upcoming experiments.If you can, monitor pH too. When I added aragonite sand to my tank years ago, I couldn’t see any alk change, but pH dropped a bit right away and that may be a more sensitive way to detect loss of carbonate.
I went ahead and bought CaribSea ARM coarse medium. not knowing how close it came to the material used 4 years ago. I will give it a try anyway and we can go from go from there, possibly identifying a source of a purer form of aragonite.
The new ARM is mined calcite. It appears phosphate free (as much as I can test) and it is fine for CaRx media with a slightly different tune (bit more co2, at least for me). No idea how it compares to the older ocean harvested aragonite.
I have a dozen bags of aragonite on hand. If you tell me how much of the sodium triphosphate to add to 10 gallons of fresh saltwater to make 50 ppm, I can put a reactor full of the aragonite on there and see where it settles out in a week.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,474
- Reaction score
- 63,874
One thing to keep in mind regarding co-precipitation of calcium carbonate and calcium phosphate is how much easier it is to detect a phosphate drop of similar size, compared to carbonate.
We can easily detect a drop of 0.1 ppm (~0.1 mg/L) phosphate, which corresponds to 0.0010 millimoles per L.
How about detecting that much carbonate via an alk change? 0.001 millimoles of carbonate/L = 0.002 meq/L of alk = 0.006 dKH. Not easily detected. Even 10x that amount (0.06 dKH) is not readily detected.
Thus, co-precipitation is hard to prove with our tools, but is likely to happen with raw salt mixes on clean sand.
We can easily detect a drop of 0.1 ppm (~0.1 mg/L) phosphate, which corresponds to 0.0010 millimoles per L.
How about detecting that much carbonate via an alk change? 0.001 millimoles of carbonate/L = 0.002 meq/L of alk = 0.006 dKH. Not easily detected. Even 10x that amount (0.06 dKH) is not readily detected.
Thus, co-precipitation is hard to prove with our tools, but is likely to happen with raw salt mixes on clean sand.
Last edited:
- Joined
- Sep 21, 2018
- Messages
- 6,696
- Reaction score
- 7,184
Let me mull over that very generous offer. I would like to finish up the current experiment which could clarify phosphate adsorption a little and tell us where the holes are in our data.The new ARM is mined calcite. It appears phosphate free (as much as I can test) and it is fine for CaRx media with a slightly different tune (bit more co2, at least for me). No idea how it compares to the older ocean harvested aragonite.
I have a dozen bags of aragonite on hand. If you tell me how much of the sodium triphosphate to add to 10 gallons of fresh saltwater to make 50 ppm, I can put a reactor full of the aragonite on there and see where it settles out in a week.


Dan
Similar threads
- Replies
- 9
- Views
- 198
- Replies
- 6
- Views
- 171
- Replies
- 16
- Views
- 362
- Replies
- 66
- Views
- 1,295
- Replies
- 1
- Views
- 194
















