I don’t think that a little phosphate in the sand has been proved to be bad, may be the opposite. I plan on using a small sample of this phosphate soaked sand for a couple of growth experiments, eventually.Dan whats your opinion on this data repeat pattern I'm seeing
tap water is supposed to be variable/high in phosphates and impurities
we have so many uncountable rip clean threads all using tap and I give you my word nobody has ever pm'd me or skewered the thread one time in all these years about us ruining their substrate with tap water...causing it to become fertilizer for plants that it wasn't before. rip cleans have about 90% rate testimony for helpful prevention of algae and sandbed matted invaders, a 10% neutral/no change/same frequency of invasion report, and a zero pct report rate of a tap water rinse on sandbed for 3 hours making things worse.
is it contact time? things added into a reef tank are sinked and staying, maybe three hours doesn't transmit much phosphate for the bind into the matrix?
perhaps we do give em a lil phosphate for the uptake but the actual impact of it changes, chemically along the way before plant or moneran mat uptake at a higher rate
perhaps we give them phosphate but the net benefit of ripping out all the leaking organic stores outweighs it
some detail of exposing sands to high phosphate water/ three hour increments on average isn't really amounting to much - impact. its all +
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Aragonite Sand And Phosphate Adsorption
- Thread starter Dan_P
- Start date
- Tagged users None
Indeed, mine is trying to replicate a sand bed. Not sure how bacterial interactions in a running tank would mess with that, however. I do have lots of new sand left over, lolA couple technical notes. Calcium carbonate might never reach an equilibrium but adsorption does slow down to a very boring rate.
For a well mixed pile of sand - think sand storm in a beaker - about 70% of the amount bound in a twenty four hour period is on the sand by one hour. The other 30% binds in 23 hours. Adsorption continues after that.
For your system, the sand is not near saturation and will continue to bind phosphate. You are about 10% or so of your target.
For your less than well stirred system, phosphate adsorption is slowed by diffusion through the sand. Where a well stirred system nears completion at twenty four hours, an unstirred systems could take weeks.
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
No simple answer here. Temperature, pH, PO4 concentration, phosphate content of the sand and sand grain size determines whether the sand loses or gains phosphate. Three hours is enough time to add to or subtract from phosphate in the sand if other conditions are good for adsorption.Dan whats your opinion on this data repeat pattern I'm seeing
tap water is supposed to be variable/high in phosphates and impurities
we have so many uncountable rip clean threads all using tap and I give you my word nobody has ever pm'd me or skewered the thread one time in all these years about us ruining their substrate with tap water...causing it to become fertilizer for plants that it wasn't before. rip cleans have about 90% rate testimony for helpful prevention of algae and sandbed matted invaders, a 10% neutral/no change/same frequency of invasion report, and a zero pct report rate of a tap water rinse on sandbed for 3 hours making things worse.
is it contact time? things added into a reef tank are sinked and staying, maybe three hours doesn't transmit much phosphate for the bind into the matrix?
perhaps we do give em a lil phosphate for the uptake but the actual impact of it changes, chemically along the way before plant or moneran mat uptake at a higher rate
perhaps we give them phosphate but the net benefit of ripping out all the leaking organic stores outweighs it
some detail of exposing sands to high phosphate water/ three hour increments on average isn't really amounting to much - impact. its all +
If we can agree on typical values for rip cleaning temperature, pH, wash water phosphate concentration, I can rip clean a sample of sand, either clean or saturated with phosphare, !and look for a phosphate change.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,757
- Reaction score
- 65,472
Dan whats your opinion on this data repeat pattern I'm seeing
tap water is supposed to be variable/high in phosphates and impurities
we have so many uncountable rip clean threads all using tap and I give you my word nobody has ever pm'd me or skewered the thread one time in all these years about us ruining their substrate with tap water...causing it to become fertilizer for plants that it wasn't before. rip cleans have about 90% rate testimony for helpful prevention of algae and sandbed matted invaders, a 10% neutral/no change/same frequency of invasion report, and a zero pct report rate of a tap water rinse on sandbed for 3 hours making things worse.
is it contact time? things added into a reef tank are sinked and staying, maybe three hours doesn't transmit much phosphate for the bind into the matrix?
perhaps we do give em a lil phosphate for the uptake but the actual impact of it changes, chemically along the way before plant or moneran mat uptake at a higher rate
perhaps we give them phosphate but the net benefit of ripping out all the leaking organic stores outweighs it
some detail of exposing sands to high phosphate water/ three hour increments on average isn't really amounting to much - impact. its all +
A few comments...
While I have used tap water for washing sand in the past, I'm not of the opinion that it is a risk free endeavor. I would not recommend it in general unless one knew something about the tap water. Copper is a concern in addition to phosphate, IMO.
Sand that is so loaded with organics that one feels a need to take it out and rinse it likely has a lot less surface available for phosphate binding because organics are taking up many of the same sites. So the risk may be lower on live sand than new sand.
It also may already have substantial phosphate on it from the degrading organics.
But we also need to take into account the mass of phosphate available in the tap water that is used. When I have rinsed sand, I rinsed multiple pounds of oolitic aragonite in less than 10 gallons of water, spread out over several rinse batches.
Worst case, how much is that?
I saw a report from the water company that NYC had up to 5 ppm (5 mg/L) phosphate (used in my old tap water article, no idea if they still do this). Let's say I washed 5 pounds of sand in 10 gallons of that water. That 10 gallons (38 L) contains about 190 mg of phosphate.
That means we have 5 pounds (2268 grams) of sand interacting with190 mg of phosphate, or the equivalent of 0.76 g phosphate per 20 pounds.
Dan posted this graphic in the first post of this thread:
Thus, in a worst case, it is more than enough to load up the sand by Dan's experiment, but not by JDA's or Millero's on different materials.
However, many people may have tap water may have far less phosphate than 5 ppm. Mine reported 0.009 ppm in June 2022.
Thus, the issue of rinsing sand is, IMO, just like the issue of using tap water directly. It may work for many people, but it may not work for everyone.
but we didnt know that until we hit the ten thousandth rip clean mark
and in that hindsight I've not seen one reported loss of invert/much less a pattern and that's a consequence expected for copper loading
we see only stark reduction in algae issues vs increase/the consequence of po4 loading expected
the chemistry expected is wildly consequence free and its not even with outliers present, if there are some they never reported back. I have never had any feedback whatsoever of any type of damage from copper or phosphate loading although N=10000 isn't a landslide like n=100000/need more time to log results
People love to wreck big work threads with noncompliance reports...consequence for the author time...I feel this collective data on its 9th year now and all happy posters forces us to reconsider consequence prediction given the known chemistry
and in that hindsight I've not seen one reported loss of invert/much less a pattern and that's a consequence expected for copper loading
we see only stark reduction in algae issues vs increase/the consequence of po4 loading expected
the chemistry expected is wildly consequence free and its not even with outliers present, if there are some they never reported back. I have never had any feedback whatsoever of any type of damage from copper or phosphate loading although N=10000 isn't a landslide like n=100000/need more time to log results
People love to wreck big work threads with noncompliance reports...consequence for the author time...I feel this collective data on its 9th year now and all happy posters forces us to reconsider consequence prediction given the known chemistry
in contrast we have several consequence wiped tanks from incomplete sandbed rinsing, or skip rinsing where tank transfers simply moved over the massive waste sink that is any unrinsed sandbed sitting stratified and locked into place on top of glass (contrasted to pauls rugf highly oxygenated pass through)
-cosmetic clouds like below/mere silt from new sand doesn't kill it's just annoying. this is 50% of the reason we rinse.
the other 50% is to prevent actual tank loss and fish kill from whatever factor exists in people's sandbeds as clouding.
some combination of irritants/lysed cells/X? is present in detritus clouds perhaps influenced by various states of oxygen and impaction per variables across tanks
using tap water allows the reefer no hesitation/no running out of water and that makes them all win...rinsing long enough to evacuate the cloud we know that carries the loss variable in substrate move jobs.
all consequence aligns with rotting mixes of organics and bacteria cells/the things that cloud when substrates are disturbed... we control all tank losses in tank transfers, upgrades and 90% +/- for invasions control specifically by rinsing in tap and no other threads exist with 100% pass rate on that many jobs using alternate means
RO water for example/ known safe for rinsing/ we don't think it's worth the work and $ to veer off course from perfect results so far with free tap water. the risk of under rinsing isn't worth the effort to keep pure safe water on hand for bulk rinsing
***how about someone stop making sand and selling it to us as no rinse needed ha ha the ultimate marketing lie.
we'd be put out of business if caribsea simply honored what is says on the bag label / use the flocculant no rinse
what keeps us in business is folks that follow directions and get this:

this is one reason I think anecdote in very very very large pattern sets is viable science from reef forums. even if the author is full of bias in assessment there's patterns trained viewers can scope from 10000 reefs doing the exact same move to discern their personal consequence % risk rate
-cosmetic clouds like below/mere silt from new sand doesn't kill it's just annoying. this is 50% of the reason we rinse.
the other 50% is to prevent actual tank loss and fish kill from whatever factor exists in people's sandbeds as clouding.
some combination of irritants/lysed cells/X? is present in detritus clouds perhaps influenced by various states of oxygen and impaction per variables across tanks
using tap water allows the reefer no hesitation/no running out of water and that makes them all win...rinsing long enough to evacuate the cloud we know that carries the loss variable in substrate move jobs.
all consequence aligns with rotting mixes of organics and bacteria cells/the things that cloud when substrates are disturbed... we control all tank losses in tank transfers, upgrades and 90% +/- for invasions control specifically by rinsing in tap and no other threads exist with 100% pass rate on that many jobs using alternate means
RO water for example/ known safe for rinsing/ we don't think it's worth the work and $ to veer off course from perfect results so far with free tap water. the risk of under rinsing isn't worth the effort to keep pure safe water on hand for bulk rinsing
***how about someone stop making sand and selling it to us as no rinse needed ha ha the ultimate marketing lie.
we'd be put out of business if caribsea simply honored what is says on the bag label / use the flocculant no rinse
what keeps us in business is folks that follow directions and get this:
this is one reason I think anecdote in very very very large pattern sets is viable science from reef forums. even if the author is full of bias in assessment there's patterns trained viewers can scope from 10000 reefs doing the exact same move to discern their personal consequence % risk rate
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,757
- Reaction score
- 65,472
but we didnt know that until we hit the ten thousandth rip clean mark
Have you seen any reported loses that are definitively from elevated phosphate?
no and I truly don't have any losses attributed to anything
we had a fish jump out of holding once...in all the bulk pages:

 www.reef2reef.com
www.reef2reef.com
of course that's me opining for nine years lol but the scoopable part is I've been asking for before and after pics consistently the whole time and posters are free to update
knowing outcome bias is likely I wanted to leave a simple pattern set of take apart reef, separate all animals from cloudable waste, rinse sand in tap water until a drop test into a clean glass of water = snowglobe clean grains
then track the rest of the tank's life arc to see what happens. all via pic updates. we can select their post history on the early pages back from 2016 to see how those tanks fared up to today/if still updating. some very minable data is available in the opinion set above that's for sure. I don't want them to use ro because it could run out, leaving us at 1/4 cloud
if anyone's tank dies I'd consider the method faulty and would quit advising the full tap rinse. only partial rinses scare me, for whatever reason they may be partially rinsing (some do it to leave a little bacteria in the tank as a seed, others may opt to use RO water to avoid contaminants then they run out of rinse water too quickly)
I'm 100% sure a cloudless tap rinse of sand stops all mini and macro cycles in reef tank substrate transfer. *we may be very well exposed to copper and phosphate, all I can do is keep the data train growing to see what happens over the years. fun test of the null hypothesis anyway...
we had a fish jump out of holding once...in all the bulk pages:

Official Sand Rinse and Tank Transfer thread
If you are reading this thread to cure a tank invasion from a link I sent you, we do not need to identify your type of invasion here we do not need you to test anything at anytime regarding nitrate, phosphate etc Above all, we do not need to see a microscope slide picture of your invasion at...
 www.reef2reef.com
www.reef2reef.com
of course that's me opining for nine years lol but the scoopable part is I've been asking for before and after pics consistently the whole time and posters are free to update
knowing outcome bias is likely I wanted to leave a simple pattern set of take apart reef, separate all animals from cloudable waste, rinse sand in tap water until a drop test into a clean glass of water = snowglobe clean grains
then track the rest of the tank's life arc to see what happens. all via pic updates. we can select their post history on the early pages back from 2016 to see how those tanks fared up to today/if still updating. some very minable data is available in the opinion set above that's for sure. I don't want them to use ro because it could run out, leaving us at 1/4 cloud
if anyone's tank dies I'd consider the method faulty and would quit advising the full tap rinse. only partial rinses scare me, for whatever reason they may be partially rinsing (some do it to leave a little bacteria in the tank as a seed, others may opt to use RO water to avoid contaminants then they run out of rinse water too quickly)
I'm 100% sure a cloudless tap rinse of sand stops all mini and macro cycles in reef tank substrate transfer. *we may be very well exposed to copper and phosphate, all I can do is keep the data train growing to see what happens over the years. fun test of the null hypothesis anyway...
Last edited:
The wife rinsed the sand in her new tank, in tap with 1ppm phosphate, then filled with 90% new water, 10% water from my tank. Nothing odd showed up, just 10% of my tank water. I’ve got a copper test somewhere.*we may be very well exposed to copper and phosphate
Edit - when I say nothing odd, this thread suggests phosphate should have gone mega low, and stayed there, it didn’t which is baffling me to be honest. Perhaps the rinsing did load it up? One thing for sure, there’s no Dino outbreak in the tank.
Last edited:
- Joined
- Jan 19, 2020
- Messages
- 1,381
- Reaction score
- 1,845
So if I had a stable Phosphate and vacuum all my sand or stirred it up, would it then cause a shift in phosphate?For your less than well stirred system, phosphate adsorption is slowed by diffusion through the sand. Where a well stirred system nears completion at twenty four hours, an unstirred systems could take weeks.
Depending on the system, phosphate could go up or down?
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
Theoretically, yes. I have done exactly one experiment so farSo if I had a stable Phosphate and vacuum all my sand or stirred it up, would it then cause a shift in phosphate?
Depending on the system, phosphate could go up or down?
I mixed a sample of sand from the surface of my sand bed with Instant Ocean and observed phosphate increase in the Instant Ocean. I imagine if the phosphate level in pore water is higher than the water above the sand bed (I have measured this), then bringing the deep sand up to the surface would result in the release of some phosphate.
I’ve given it another week of not dosing and got a similar result to last week at around 0.1ppm so I guess it’s done. Resumed dosing just now, added 4ppm to make up a bit of timeI'd wait a few days, retest the water, and if it hasn't dropped, wait more and do a final test.
If it seems to drop a bit then keep watching as long as it does.
Merry Winter Solstice Festival folks. My little test is consuming 3ppm every 48 hrs by the looks of it. Can anyone see why I shouldn’t keep bumping the Phos upto 4ppm between tests? it’s speeding up things by quite a bit, cheers
Last edited:
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
No reasons come to mind because for sand, there does not seem to be much of adsorption difference with increasing PO4 concentration, though you might be speeding up the process of saturating the sand.Merry Winter Solstice Festival folks. My little test is consuming 3ppm every 48 hrs by the looks of it. Can anyone see why I shouldn’t keep bumping the Phos upto 4ppm between tests? it’s speeding up things by quite a bit, cheers
One observation that seems to be solidifying into fact is that sand accumulates PO4 that does not come off. I stumbled onto this in the study above (see plot below) but some thought observation meant the sand in the experiment was not equilibrating long enough. Subsequent experiments to be posted seem to rule this out. So, one possibility is that aragonite might go on slowly adsorbing PO4 for quite some time. Slowly means below the detection of hobby tests but maybe of importance to nuisance algae. Fun fact. Since my last post, I have used nearly 300 packets of Hanna PO4 reagent. Working towards 400 pretty quickly.
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
A philosophical response this time.Merry Winter Solstice Festival folks. My little test is consuming 3ppm every 48 hrs by the looks of it. Can anyone see why I shouldn’t keep bumping the Phos upto 4ppm between tests? it’s speeding up things by quite a bit, cheers
When exposing sand to 4 ppm PO4, the experiment is in a region that might be consider outside the range of PO4 concentrations that aquarium sand is ever exposed to. If we saturate the sand with these conditions, it is being saturated at PO4 concentrations the sand will never encounter. So what has this experiment shown? I ask this question of myself too. After spending weeks understanding PO4 adsorption in the 0.2 ppm and above range, it dawned on me that our aquaria water usually sit at <0.1 ppm
Indeed, and covered in other “stuff”. I maybe wasting my time, wouldn’t be the first time, lolit dawned on me that our aquaria water usually sit at <0.1 ppm
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
Investigations often have many blind alleys. Appreciating delayed gratification is keyIndeed, and covered in other “stuff”. I maybe wasting my time, wouldn’t be the first time, lol
Wow, nice inducement to carry on, you’re good, very goodInvestigations often have many blind alleys. Appreciating delayed gratification is key
- Joined
- Sep 21, 2018
- Messages
- 6,982
- Reaction score
- 7,438
Aragonite Sand And Phosphate Adsorption. Sand Grain Size Study
The data I reported above raised some questions that needed addressing, especially, “why were my results so different from published data”. I have new data that provides a plausible answer: surface area.
@Randy Holmes-Farley has made clear that surface area is an important factor in determining the phosphate adsorbing capacity of calcium carbonate. Taking the hint, I ground my aragonite sand in a mortar and pestle, and than with a 200 mesh sieve, isolated sand grains that were comparable to those used by Millero, et al. The plot below (with its hobby friendly units) shows Millero’s results (green), and my results for sugar sand (orange), and ground and sieved sugar sand (blue). The match between Millero’s results and mine for ground sand is not perfect but close. A possible reason for the curve shape difference is mineral content. Millero used pure aragonite whereas I used aragonite sand which is likely a mixture of calcite and aragonite. In fact, my results resembles Millero‘s results for calcite. By the way, the idea of adding powdered calcium carbonate to remove phosphate from the water (but maybe not from the aquarium) gets a boost in credibility from this data, though we are likely adding the lower binding capacity mineral calcite.
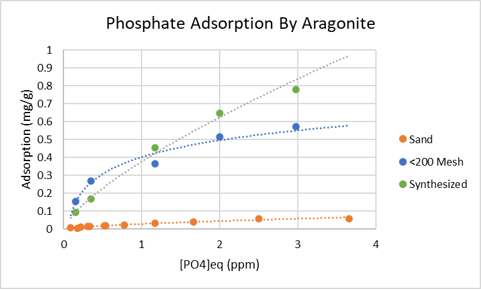
The data I reported above raised some questions that needed addressing, especially, “why were my results so different from published data”. I have new data that provides a plausible answer: surface area.
@Randy Holmes-Farley has made clear that surface area is an important factor in determining the phosphate adsorbing capacity of calcium carbonate. Taking the hint, I ground my aragonite sand in a mortar and pestle, and than with a 200 mesh sieve, isolated sand grains that were comparable to those used by Millero, et al. The plot below (with its hobby friendly units) shows Millero’s results (green), and my results for sugar sand (orange), and ground and sieved sugar sand (blue). The match between Millero’s results and mine for ground sand is not perfect but close. A possible reason for the curve shape difference is mineral content. Millero used pure aragonite whereas I used aragonite sand which is likely a mixture of calcite and aragonite. In fact, my results resembles Millero‘s results for calcite. By the way, the idea of adding powdered calcium carbonate to remove phosphate from the water (but maybe not from the aquarium) gets a boost in credibility from this data, though we are likely adding the lower binding capacity mineral calcite.
Similar threads
- Replies
- 4
- Views
- 323
- Replies
- 9
- Views
- 370
- Replies
- 10
- Views
- 452
- Replies
- 6
- Views
- 278
- Replies
- 16
- Views
- 518

















