MnSO₄ - 4H₂O, thanksZinc sulfate is 40% zinc by weight.
Is the manganese sulfate hydrated?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
diy iron and manganese supplement
- Thread starter Swip
- Start date
- Tagged users None
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
MnSO₄ - 4H₂O, thanks
That product is 25% manganese by weight.
Dissolve 1 gram of the solid material in 1 L of RO/DI. That solution contains 250 mg of manganese, so the concentration is 250 mg/L or 250 ug/mL.
Adding 1 mL to 250 L of tank water will boost aquarium manganese levels by 250 ug per 250 L or 1 ug/L.
Thank you Randy, you are geniusFor the zinc sulfate, dissolve 1 gram of the solid material in 1 L of RO/DI. That solution contains 400 mg of zinc, so the concentration is 400 mg/L or 400 ug/mL.
Adding 1 mL to 400 L of tank water will boost aquarium zinc levels by 400 ug per 100 L or 4 ug/L.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
That calculation wouldn't show it, but I don't deny it to be true. 
Happy reefing!
Happy reefing!
Hi Randy,
I've been reading your threads and I must say they are super helpful and many thanks to you for helping us!))))
I'm wondering if you could kindly help me with the mixing two potions to dose my 1400L reef tank.
According to ICP my Manganese is o ug/l and set point is 2 ug/l (instructions indicate a correction dose performed in 2 days 14ml x2) and daily dosage of 2.33ml after wards) However I would like to make my own TBH.
I really would like to make sure my calculations are correct before I kill anything in there LOL.
I've purchased.
Manganese (II) Chloride Tetrahydrate 98%
Specification
Minimum Assay: >98%
Molecular Formula: Cl2Mn•4H2O
Molecular Weight: 197.90 (125.84anhy)
Appearance Form: crystalline
Colour: light red
pH: 4.0 - 6 at 99 g/l at 25 °C
Melting point/range: 58 °C
Relative density 1.913 g/cm3
Water solubility: 99 g/l at 20 °C - completely soluble
Another one is Iodine (I know you said it might not be useful but I just want to have this info for "in case to have situations"
My prescription is to dose my 1400L with 1.70ml Daily or Triton iodine
I've got,
Potassium Iodide 99.9% ACS
Specification
Minimum Assay: >99%
Molecular Formula: KI
Molecular Weight: 166.0 g/mol
Melting Point: 681 °C
Iodate (as KIO3): <0.0004%
Heavy Metals (as Pb): <0.001%
Loss on Drying: <1%
Alkalinity: Passes Test
Nitrates,Ammonia, Nitrites: Passes Test
Thiosulphate and Barium: Passes Test
Sorry for asking this but I'm not chemist and feel as if I'm going to dentist for the first time in my life)))))
Many thanks,
Anita
I've been reading your threads and I must say they are super helpful and many thanks to you for helping us!))))
I'm wondering if you could kindly help me with the mixing two potions to dose my 1400L reef tank.
According to ICP my Manganese is o ug/l and set point is 2 ug/l (instructions indicate a correction dose performed in 2 days 14ml x2) and daily dosage of 2.33ml after wards) However I would like to make my own TBH.
I really would like to make sure my calculations are correct before I kill anything in there LOL.
I've purchased.
Manganese (II) Chloride Tetrahydrate 98%
Specification
Minimum Assay: >98%
Molecular Formula: Cl2Mn•4H2O
Molecular Weight: 197.90 (125.84anhy)
Appearance Form: crystalline
Colour: light red
pH: 4.0 - 6 at 99 g/l at 25 °C
Melting point/range: 58 °C
Relative density 1.913 g/cm3
Water solubility: 99 g/l at 20 °C - completely soluble
Another one is Iodine (I know you said it might not be useful but I just want to have this info for "in case to have situations"
My prescription is to dose my 1400L with 1.70ml Daily or Triton iodine
I've got,
Potassium Iodide 99.9% ACS
Specification
Minimum Assay: >99%
Molecular Formula: KI
Molecular Weight: 166.0 g/mol
Melting Point: 681 °C
Iodate (as KIO3): <0.0004%
Heavy Metals (as Pb): <0.001%
Loss on Drying: <1%
Alkalinity: Passes Test
Nitrates,Ammonia, Nitrites: Passes Test
Thiosulphate and Barium: Passes Test
Sorry for asking this but I'm not chemist and feel as if I'm going to dentist for the first time in my life)))))
Many thanks,
Anita
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
Are you planning to use a scale to weigh these out?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
The manganese supplement is 28% manganese by weight. If you want 2 ug/ L manganese then you need to dose 7 ug/L of solids or 700 ug/ 100 L tank water.
You can’t weigh out so little, so make a standard of 0.7 grams solids per liter of RO/DI. It has 700 mg/L or 700 ug/ mL. Add 1 mL per 100 L of tank volume and you should be set.
You can’t weigh out so little, so make a standard of 0.7 grams solids per liter of RO/DI. It has 700 mg/L or 700 ug/ mL. Add 1 mL per 100 L of tank volume and you should be set.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
The potassium iodide is 77% iodide.
Dissolve 1 g in a liter of RO/Di. That solution is 770 mg/L iodide or 0.77 mg/mL.
To boost iodine by 0.03 mg/L in 100 L of tank volume, you need 3 mg of iodide, or 3/0.77 = 3.9 mL for each 100 L of tank volume.
Dissolve 1 g in a liter of RO/Di. That solution is 770 mg/L iodide or 0.77 mg/mL.
To boost iodine by 0.03 mg/L in 100 L of tank volume, you need 3 mg of iodide, or 3/0.77 = 3.9 mL for each 100 L of tank volume.
Hi Randy,
Yes, I do have some scales capable to do 0.01 gr and up, so should be ok)
Thank you, I made the same calculations but wanted to make sure that the powder I bought has this concentration as even the supplier couldn't give me proper answer.
Yes, I do have some scales capable to do 0.01 gr and up, so should be ok)
Thank you, I made the same calculations but wanted to make sure that the powder I bought has this concentration as even the supplier couldn't give me proper answer.
Last edited:
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
You’re welcome. Happy Reefing [emoji3]
- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
There are just a few things you need to know to calculate stuff. I made a table to make this clear for myself.Hi Randy,
Yes, I do have some scales capable to do 0.01 gr and up, so should be ok)
Thank you, I made the same calculations but wanted to make sure that the powder I bought has this concentration as even the supplier couldn't give me proper answer.
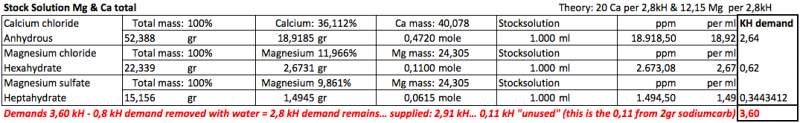
Is there a formula for manganese sulfate?Yes.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
Is there a formula for manganese sulfate?
Post 42 of this thread discusses manganese sulfate.
I found some nickel sulfate. But the handling and storage of nickel and sodium fluoride is steering me away from DIYing these 2. Not wanting to buy a bomb suit lol. Any alternatives?Unless you are very into experimentation, I wouldn't boost nickel in a reef tank. Triton can only just barely detect the natural level, so none detected does not prove a big deficiency, and I'm not sure it has a beneficial role in reef tanks anyway. It can be toxic if overdosed.
Thanks for posting these, RHF. I'm off to the store today to pick up some iron and manganese per your chemistry archive mixing instructions.Manganese(II) chloride tetrahydrate is 28% manganese by weight.
Here's a recipe:
Dissolve 1 gram in 100o mL (grams) fresh water. Manganese = 280 ppm.
Take 1 mL (1 g) of that mix and dissolve in 1000 mL of fresh water. Mn = 0.28 ppm (=280 ug/L).
Add 1 mL of that to 100 mL of fresh water. Mn = 2.8 ug/L
Add 13.5 mL of that to 100 gallons of tank water. Conc boost to tank = 0.1 ug/L Mn.
For the Mn, can we stop at the second to last step to create a more concentrated stock with a goal to dose less daily volume? Stopping at 1.35 mL to 100 gallons of tank water to provide a 0.1 ug/L at the Mn = 2.8 ug/L step, or is there solubility issue or another reason to dilute it further?
Thank you
MnSO₄ - 4H₂O, thanks
That product is 25% manganese by weight.
Dissolve 1 gram of the solid material in 1 L of RO/DI. That solution contains 250 mg of manganese, so the concentration is 250 mg/L or 250 ug/mL.
Adding 1 mL to 250 L of tank water will boost aquarium manganese levels by 250 ug per 250 L or 1 ug/L.
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Can I use the manganese sulfate monohydrated? The math is the same?
Thanks
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
That product is 25% manganese by weight.
Dissolve 1 gram of the solid material in 1 L of RO/DI. That solution contains 250 mg of manganese, so the concentration is 250 mg/L or 250 ug/mL.
Adding 1 mL to 250 L of tank water will boost aquarium manganese levels by 250 ug per 250 L or 1 ug/L.
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------
Can I use the manganese sulfate monohydrated? The math is the same?
Thanks
Similar, but not identical. The mini hydrate will give slightly higher potency.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
Thanks for posting these, RHF. I'm off to the store today to pick up some iron and manganese per your chemistry archive mixing instructions.
For the Mn, can we stop at the second to last step to create a more concentrated stock with a goal to dose less daily volume? Stopping at 1.35 mL to 100 gallons of tank water to provide a 0.1 ug/L at the Mn = 2.8 ug/L step, or is there solubility issue or another reason to dilute it further?
Thank you
If the solution is clear, you can use any dilution you like.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,451
- Reaction score
- 63,845
Note that the last dilution us 100x, not 10x, so it will add tens times more then you calculated.
Same thing, working backwards x=(.02 mg/l *1,000,000 ml)/(2.5 ml) gives the 1L stock solution a 8000 ppm. but when then I try to adjust for a 225 gallon tank (~852L) I can seem to get your value. Based on the equation above I need 21.30 ml to dose however (8000 mg/l *21.3 ml) = (x * 852,000). x= (8000 mg/l * 21.30 ml)/(852,000 ml) gives me a ppm of 0.2. Am I doing something wrong?Potassium iodide is 77% iodine by weight.
If you target 0.02 ppm to dose, then you will want to add 0.02 mg iodide per liter of aquarium water.
That means dosing 0.025 mg of the KI per liter or 2.5 mg/100 L of aquarium water.
You won't likely be able to weight that small of an amount, so make a stock dosing solution.
Dissolve 1 g KI in 1 L RO/DI. That solution contains 1 mg KI per mL.
So 2.5 mL of that solution added per 100 L of aquarium water will boost iodine by 0.02 ppm.
I think you can dose this at least a couple of times a week in a typical tank.
That all said, I'm also not of the opinion that iodine dosing is useful for most reef tanks.
Similar threads
-
- AMS: Article
- Replies
- 96
- Views
- 5,966
-
- AMS: Article
- Replies
- 287
- Views
- 12,995
- Replies
- 6
- Views
- 402

















