Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
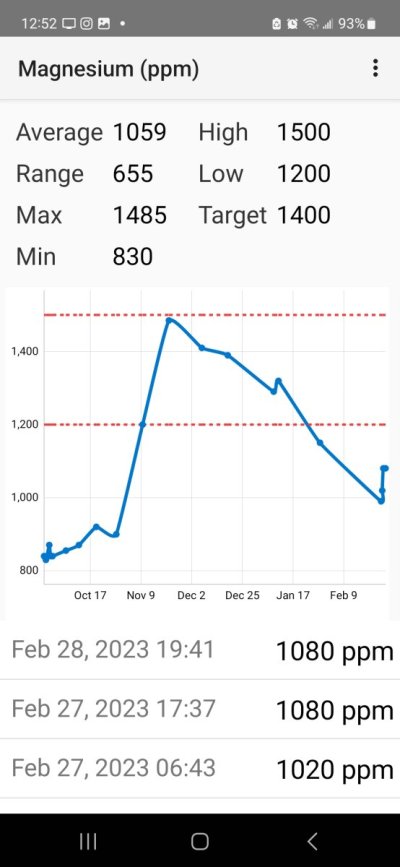
EXTREME Magnesium Consumption
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,311
- Reaction score
- 64,805
Im becoming concerned this is a troll thread that neither I nor any readers want to waste our time on.
The things you are posting cannot happen and, if they are true observations, are test error.
If this is not a troll thread, I think you should stop testing magnesium, and just focus on other reef tasks. Do not believe that your tank is the one tank in the reefing universe that has massive unexplained and sometimes instantaneous drops in magnesium that have no possible chemical explanation.
If you truly cannot stop yourself, measure magnesium in a batch of new salt water, verify it is ok, and do a complete water change.
The things you are posting cannot happen and, if they are true observations, are test error.
If this is not a troll thread, I think you should stop testing magnesium, and just focus on other reef tasks. Do not believe that your tank is the one tank in the reefing universe that has massive unexplained and sometimes instantaneous drops in magnesium that have no possible chemical explanation.
If you truly cannot stop yourself, measure magnesium in a batch of new salt water, verify it is ok, and do a complete water change.
Last edited:
Upvote
0
The thought had crossed my mind...Im becoming concerned this is a troll thread that neither I nor any readers
Upvote
0
It's not a troll. My LFSs have been far more helpful than you guys. All you guys have done is doubt. They can test the water and see what I'm saying is true and they are able to provide me with far better help than "that's impossible". In the past few days,I've gotten so much practice at testing because I can now get within 10ppm variance on even my salifert kits. I can use the aquaforest kits to check because they come with the reference solution. So I know I'm not far off on my testing. Thanks for nothing I suppose.Im becoming concerned this is a troll thread that neither I nor any readers want to waste our time on.
The things you are posting cannot happen and, if they are true observations, are test error.
If this is not a troll thread, I think you should stop testing magnesium, and just focus on other reef tasks. Do not believe that your tank is the one tank in the reefing universe that has massive unexplained and sometimes instantaneous drops in magnesium that have no possible chemical explanation.
If you truly cannot stop yourself, measure magnesium in a batch of new salt water, verify it is ok, and do a complete water change.
Upvote
0
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,311
- Reaction score
- 64,805
“Thanks for nothing I suppose.”
Well, you will find one day that we were right, and while I do not know what the lfs did that was useful, I can absolutely assure you that they do not know some secret magnesium chemistry that we do not.
I have spent a massive amount of time trying to help you. Thank you for nothing tells me I am done and am no longer going to give you expert chemistry advice.
Well, you will find one day that we were right, and while I do not know what the lfs did that was useful, I can absolutely assure you that they do not know some secret magnesium chemistry that we do not.
I have spent a massive amount of time trying to help you. Thank you for nothing tells me I am done and am no longer going to give you expert chemistry advice.
Last edited by a moderator:
Upvote
0
There is no secret. One part is that there's a strain of coraline algae in our area that consumes very high amounts of magnesium. They said unfortunately the consumption is normal due to my small water volume. They've had to find ways around it but have an easier time due to the larger systems giving a larger buffer. Also, I overhauled my RODI station even though tds out was already 0 and have noticed better stability in perms. A theory I have is that, my mixing station next to a heat source (heating the water) I would test shortly after the recommended mixing period so everything would appear fine. However, after sitting, heat, caused Calc, mag and alk to drop. Unlikely l, but not impossible with some brands. Next, you may possibly partially right about the testing error but no fault of my own. This is a stretch but I will have to wait to get the ICP test back to see and to run my own experiments. Aluminum bonds to OH to create Al(OH)3 and bonds to Cl in HCL based test to create AlCl3. Considering many titration tests are NaOH or HCL based there's a possibility that they are interfering. Red Sea AB+ is full of aluminum. It would explain poor coral health and wonky testing. Tests work fine out of tank then they give false lows once the high aluminum contaminated tank water. Also unlikey. I think I'm going to take my chances and log off of here for the last time.“Thanks for nothing I suppose.”
Well, you will find one day that we were right, and while I do not know what the lfs did that was useful, I can absolutely assure you that they do not know some secret magnesium chemistry that we do not.
I have spent a massive amount of time trying to help you. Your thank you for nothing tells me I am done and am no longer going to give you expert chemistry advice.
Upvote
0
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,311
- Reaction score
- 64,805
There is no secret. One part is that there's a strain of coraline algae in our area that consumes very high amounts of magnesium. They said unfortunately the consumption is normal due to my small water volume.
Well, you get more free advice because I do not want others misled by that commentary: that LFS comment is TOTALLY ludicrous and wrong.
Unlike your LFS, I know exactly how much magnesium, calcium, and alkalinity is consumed proportionally by coralline. I have written articles on it:

Aquarium Chemistry: Magnesium In Reef Aquaria
This article details the nature of magnesium in seawater, how it is added, measured, and removed from marine aquaria, and how it impacts the maintenance of calcium and alkalinity.
The ratio consumed by coralline is about 10 times as much calcium as magnesium. So to drop magnesium by 50 ppm in a day would consume 500 ppm of calcium.
But more importantly, the amount of alk that would be consumed is unbelievably high and unavailable: about 74 dKH.
So no, coralline is not causing more than about 2 ppm per day of magnesium consumption.
Upvote
0
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,311
- Reaction score
- 64,805
Even if you found a secret way to precipitate pure magnesium carbonate, that takes 11.5 dKH of alk per 50 ppm of magnesium.
Upvote
0
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,311
- Reaction score
- 64,805
A theory I have is that, my mixing station next to a heat source (heating the water) I would test shortly after the recommended mixing period so everything would appear fine. However, after sitting, heat, caused Calc, mag and alk to drop. Unlikely l, but not impossible with some brands.
Same problem with that theory: not enough alk and calcium present. Impossible.
This is a stretch but I will have to wait to get the ICP test back to see and to run my own experiments. Aluminum bonds to OH to create Al(OH)3 and bonds to Cl in HCL based test to create AlCl3. Considering many titration tests are NaOH or HCL based there's a possibility that they are interfering. Red Sea AB+ is full of aluminum. It would explain poor coral health and wonky testing. Tests work fine out of tank then they give false lows once the high aluminum contaminated tank water. Also unlikey. I think I'm going to take my chances and log off of here for the last time.
Too much stretch. Aluminum at any level attained in a reef tank does not do what you are claiming.
Upvote
0
Sigh. Randy is giving you the best possible help that one can expect.There is no secret. One part is that there's a strain of coraline algae in our area that consumes very high amounts of magnesium. They said unfortunately the consumption is normal due to my small water volume. They've had to find ways around it but have an easier time due to the larger systems giving a larger buffer. Also, I overhauled my RODI station even though tds out was already 0 and have noticed better stability in perms. A theory I have is that, my mixing station next to a heat source (heating the water) I would test shortly after the recommended mixing period so everything would appear fine. However, after sitting, heat, caused Calc, mag and alk to drop. Unlikely l, but not impossible with some brands. Next, you may possibly partially right about the testing error but no fault of my own. This is a stretch but I will have to wait to get the ICP test back to see and to run my own experiments. Aluminum bonds to OH to create Al(OH)3 and bonds to Cl in HCL based test to create AlCl3. Considering many titration tests are NaOH or HCL based there's a possibility that they are interfering. Red Sea AB+ is full of aluminum. It would explain poor coral health and wonky testing. Tests work fine out of tank then they give false lows once the high aluminum contaminated tank water. Also unlikey. I think I'm going to take my chances and log off of here for the last time.
Where are you located in Canada? If you are close to me I would be willing to help in person.
As mentioned by Biom, I would double check salinity and the AWC. On multiple occasions I have had an out of balance AWC (continuous) mess up my salinity and lower or raise parameters. In these cases, a no calibration required salinity tool like a TM High Precision Hydrometer comes in handy.
Upvote
0
There is no secret. One part is that there's a strain of coraline algae in our area that consumes very high amounts of magnesium.
You will find many people that will tell you exactly what you want to hear if they can make money from it. Don't chase monsters, don't spend more $$$ on this LFS, take you GF out and have some fun - she made Mg tests for you (!) that' s precious.
Upvote
0
Similar threads
- Question
- Replies
- 8
- Views
- 632
- Replies
- 8
- Views
- 327
- Replies
- 26
- Views
- 1,119
- Replies
- 23
- Views
- 919
-
- AMS: Article
- Replies
- 64
- Views
- 5,007