- Joined
- May 22, 2016
- Messages
- 6,636
- Reaction score
- 10,252
One Last update to the Biospira Temp extreme side quest
(earlier results below)
The additional result is that even the biospira sample that got hard frozen (0F) overnight, thawed, shaken refrozen, thawed.... 3 cycles of hours of deep freeze at 0F and full thaw and mixing.... still processed ammonia AND nitrite.
See purple data below.
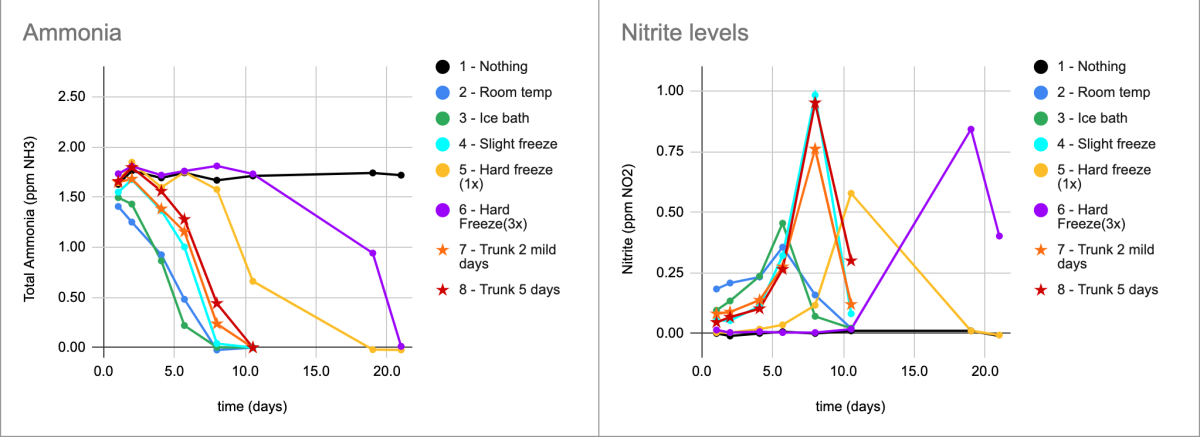
It looks like by day 17 or 18, the survivors in the sample that had been hard frozen 3x had recovered in population enough to begin visibly oxidizing ammonia and nitrite. The control that got nothing still left ammonia untouched.
Sometimes @Dan_P and I can sound like Biospira fanboys....but, good grief. The stuff is instantly active, fast working, and virtually un-killable.
I know it's a terrible business idea to tell the customer your product can't fail, but I'd be super tempted to print "idiot proof and unkillable" on the bottles.
(earlier results below)
The Biospira sample that got a single "hard freeze" in 15mL centrifuge tube overnight in the freezer at 0F - actually ended up processing ammonia AND nitrite just fine, it was merely significantly delayed. See Yellow data below.
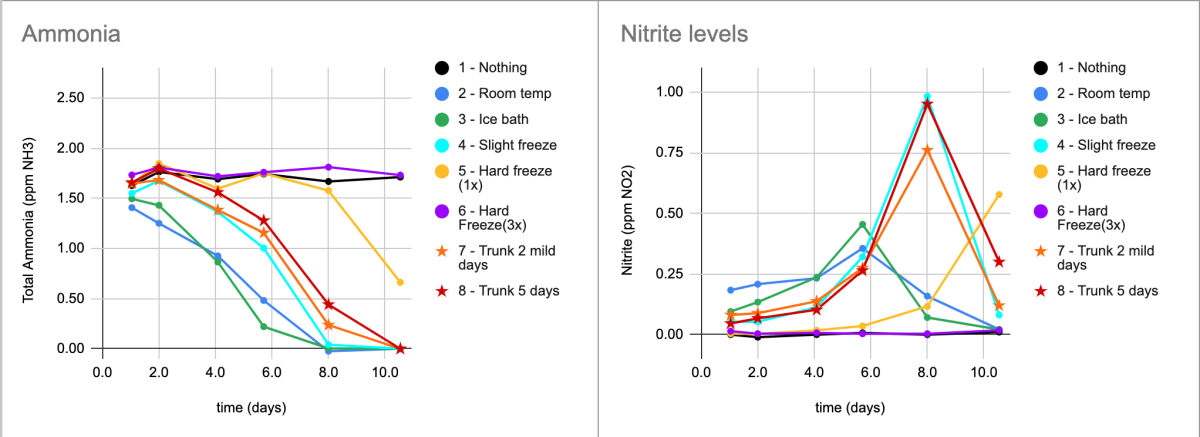
The amount of NO2-N accumulation is clearly lower than the ammonia-N consumed (like the other biospira samples) - indicating plenty of active nitrite oxidizers in this sample too. In fact, although the onset of ammonia processing was delayed by ~7 days, after that - the rate of ammonia consumed and nitrite produced looks almost identical to rate of the other active samples.
The sample that got 3x cycles of hours to overnight hard (0F) freezes and thaws still looks dead by comparison.
The additional result is that even the biospira sample that got hard frozen (0F) overnight, thawed, shaken refrozen, thawed.... 3 cycles of hours of deep freeze at 0F and full thaw and mixing.... still processed ammonia AND nitrite.
See purple data below.
It looks like by day 17 or 18, the survivors in the sample that had been hard frozen 3x had recovered in population enough to begin visibly oxidizing ammonia and nitrite. The control that got nothing still left ammonia untouched.
Sometimes @Dan_P and I can sound like Biospira fanboys....but, good grief. The stuff is instantly active, fast working, and virtually un-killable.
I know it's a terrible business idea to tell the customer your product can't fail, but I'd be super tempted to print "idiot proof and unkillable" on the bottles.




















