A MUST READ. COPY N PASTE FROM AN ARTICLE FROM RANDY HOLMES FARLEY 20 YEARS AGO
If your tank falls outside of these ranges for either or both of these measurements, then how you need to go about correcting them does indeed depend on the relationship between the two. It is this aspect of calcium and alkalinity maintenance that causes problems for many aquarists.
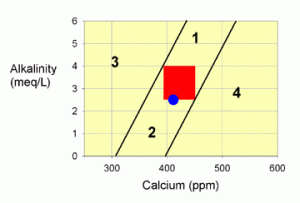
Figure 1. A graph showing possible values for calcium and alkalinity in marine aquaria. The red zone is the recommended target, and the blue dot represents values in natural seawater. Each numbered zone outside of the target area has a specific set of directions to get back to the target.
Zone 1 is the easiest problem to correct. Unfortunately, it is also very uncommon. In this case, both calcium and alkalinity are on the high side of normal. Moreover, if you leave the tank alone, the problem will likely correct itself, and you will end up in the red target zone (though you may also pass through it into zone 2 if you wait too long).
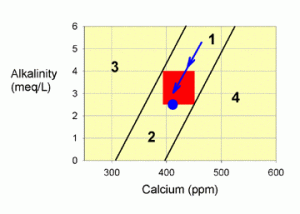
Figure 2. A graph showing how to correct values within zone 1 by allowing calcium carbonate to be deposited in the tank (the blue arrow).
What this zone implies is that both calcium and alkalinity are elevated, and that by removing calcium carbonate from the water, either through biotic deposition into coral skeletons or coralline algae, or through abiotic precipitation, as on heaters, the levels of each will drop in an appropriate ratio. More specifically, the tank parameters will move along a line parallel to the two lines bordering this zone, and directly into the red target zone (the blue arrow in Figure 2). If you are smack in the middle between these two lines, as in Figure 2, then you will continue to move in the middle of these two lines down into the target zone.
This movement can continue right out the bottom end of the target zone (into zone 2), of course, so once you reach the target zone, you’ll have to reinitiate normal calcium and alkalinity additions.
Zone 2 is also an easy problem to correct, and is very common. In this case, both calcium and alkalinity are on the low side of normal. If you take any tank in the red target zone, and let corals and coralline algae grow (i.e., calcify), then you will move into zone 2. Just as above (Figure 2), you will move downward in a fashion such that you parallel the lines bordering the zone. That fact is why it is so common to have this problem: anyone not adding enough calcium and alkalinity to balance demand in the tank will likely enter this zone.
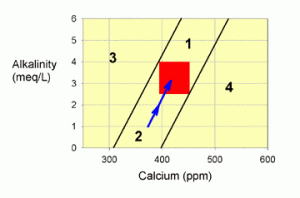
Figure 3. A graph showing how to correct values within zone 2 by supplementing with a balanced calcium and alkalinity additive system (the blue arrow).
To correct this situation back to the red target zone, one wants to add calcium and alkalinity in a balanced fashion. Fortunately, there are many ways to achieve this balance, such as with limewater (kalkwasser), calcium carbonate/carbon dioxide reactors, and the many balanced two-part calcium and alkalinity supplements (such as B-ionic, C-Balance, etc.). The more that you add of these, the further upward your correction will go (the blue arrow in Figure 3). Of course, adding too much will push you into zone 1, but if that happens you can just sit back and watch it drop back to the target zone. I’ll discuss balanced additives more at the end of the article.
Of course, you can add calcium and alkalinity that are not “balanced” (that is, not tied to one another in any specific ratio). For example, adding calcium chloride and sodium bicarbonate will work fine, but you must fine-tune exactly how much of each you add. Consequently, more careful monitoring of calcium and alkalinity levels is necessary if you go this route.
Remember that manufacturer recommendations are based on maintaining a tank, not in making substantial corrections. To make such corrections, you may need to add much more than is recommended. If you are adding a balanced additive (and the pH is not getting out of the range of about 7.9 to 8.5) then the worst that is likely to happen from overdosing (other than truly huge overdosing) is wasting some money and causing some extra precipitation of calcium carbonate on your heater and other parts of the tank. These balanced additives are discussed in more detail at the end of the article.
On the other hand, if you are using independent calcium and alkalinity additives, you must be very careful to not create an imbalance by adding too much of one relative to the other. Directions for deciding how much of these types of additives can be found below, but you should rely on frequent testing more than recommended amounts, to determine how much of these types of additives to put into the tank.
Finally, if you are adding large amount of calcium and alkalinity supplements, but just cannot maintain the desired values, you might want to measure the magnesium level in the water. Magnesium plays an important role in preventing the abiotic precipitation of calcium carbonate1, and if it is substantially depleted, you may be experiencing excessive amounts of calcium and alkalinity loss to this route. Magnesium gets the blame far more frequently, in my opinion, than it is likely responsible, but since it is easy to check with a test kit and easy to supplement if necessary, there’s no reason to not see if it is a problem. I’d advise aiming for a natural seawater level of about 1300 ppm.
Zone 3 problems are a little harder to correct, and are fairly common. It is, in fact, the problem in the real question posed at the beginning of this article (it doesn’t say so there, but the alkalinity was 3.2 meq/L). This problem is typically caused by overdosing alkalinity RELATIVE to calcium, but does not necessarily imply that calcium is either too high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.
One more word about this zone before getting to solutions: Many tanks end up here because aquarists are trying to correct pH problems by adding “buffer.” In my opinion, one should not try to correct any pH problem by simply adding an alkalinity supplement. If you are low on alkalinity, it is a fine course of action to raise the alkalinity. But if alkalinity is OK, or even high, adding an alkalinity supplement to alter the pH may simply create a worse problem. Better solutions to pH problems are discussed in this recent article6.
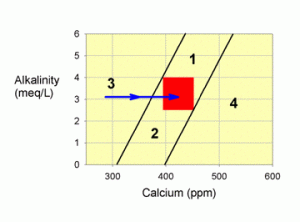
Figure 4. A graph showing how to correct values within zone 3 by adding a calcium additive, such as calcium chloride (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the right hand edge of zone 3), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
I would advise correcting this problem by adding a calcium chloride supplement until you have moved into the target zone (or zones 1 or 2 that you can then treat as described above) as shown in Figure 4. Almost any brand of calcium chloride will do (Kent Turbo Calcium, Kent Liquid Calcium, ESV, etc.). Certain other calcium supplements may also be OK (such as just the calcium component of the two-part calcium and alkalinity additive systems), but you do not want to add any alkalinity. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the edge of the zone, while you want to move over to it, and cross it.
If calcium is less than 400 ppm, I’d suggest using this handy online calculator7 to determine how much dry calcium chloride is necessary to move back to the target zone. Note that it is a minimum estimate because it does not know how much alkalinity you have, so it cannot know if you are only raising calcium directly (which it calculates) or are also precipitating calcium carbonate (when alkalinity is high this will probably happen, but is typically not a problem other than that it uses up some of what you add).
If the calcium is above 400 ppm in this zone (unlikely, but it does happen), then you can safely either do nothing until it drops and you need to add more calcium, and treat it as suggested in the previous paragraph, or you can add some calcium immediately, move into zone 1, and then just let it drop on its own.
Zone 4 problems are also a little harder to correct. It is typically caused by overdosing calcium RELATIVE to alkalinity, but does not necessarily imply that alkalinity is either to high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.
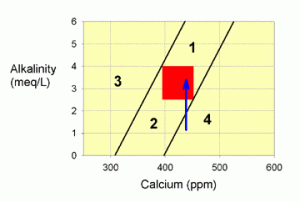
Figure 5. A graph showing how to correct values within zone 4 by adding an alkalinity additive, such as baking soda (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the left hand edge of zone 4), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
If alkalinity were less than 4 meq/L (11 dKH; the most common situation in zone 4; shown in Figure 5), I would advise correcting this problem by adding an alkalinity supplement until you have moved into the target zone (or zone 1). For systems with a pH of 8.2 or above, baking soda (sodium bicarbonate) is a good choice. For systems with a pH below 8.2, washing soda (sodium carbonate) is a good choice (though use some baking soda too if the correction is a large one and the pH gets too high; that is, above pH 8.5 or so).
In gauging how much to add, here are some rough guidelines:
Baking Soda To raise 50 gallons of tank water by 1 meq/L will require about 16 grams of baking soda (sodium bicarbonate; sodium hydrogencarbonate). Since a level teaspoon of baking soda weighs just under 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.4 meq/L (~1 dKH). Washing Soda To raise 50 gallons of tank water by 1 meq/L will require 10 grams of washing soda (sodium carbonate). Since a level teaspoon of washing soda weighs just over 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.6 meq/L (~1.7 dKH).
One special note about washing soda: Apparently some Canadian brands of washing soda contain surfactants. VIP brand, in particular, contains them and a reef keeper using them on my advice turned his tank into a bubble bath. On questioning, the manufacturer did indicate that a surfactant is present. The same reef keeper says the local Arm & Hammer brand in Canada smells strongly of perfume. I’d avoid perfumed brands, if possible. My Arm & Hammer Super Washing Soda purchased in the US apparently contains no significant surfactants, and is not perfumed. Nevertheless, anyone using washing soda for the first time ought to put some in water and stir it around to see if soapy bubbles form. If so, I’d suggest finding another brand.
Many commercial alkalinity supplements will also be fine for this purpose, as long as no significant calcium is added. In general, I don’t prefer those that contain substantial borate. The alkalinity component of the two-part calcium and alkalinity additive systems would be OK. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the left edge of the zone, while you want to move over to it, and cross it.
If alkalinity is more than 4 meq/L (11 dKH; an uncommon situation), then you can safely either do nothing until it drops and you need to add more alkalinity, and treat it as suggested in the previous two paragraphs, or you can add some alkalinity immediately, move into zone 1, and then just let it drop on its own.
Calcium and Alkalinity Problems
If your tank falls outside of these ranges for either or both of these measurements, then how you need to go about correcting them does indeed depend on the relationship between the two. It is this aspect of calcium and alkalinity maintenance that causes problems for many aquarists.

Figure 1. A graph showing possible values for calcium and alkalinity in marine aquaria. The red zone is the recommended target, and the blue dot represents values in natural seawater. Each numbered zone outside of the target area has a specific set of directions to get back to the target.
Corrections for Zone 1
Zone 1 is the easiest problem to correct. Unfortunately, it is also very uncommon. In this case, both calcium and alkalinity are on the high side of normal. Moreover, if you leave the tank alone, the problem will likely correct itself, and you will end up in the red target zone (though you may also pass through it into zone 2 if you wait too long).

Figure 2. A graph showing how to correct values within zone 1 by allowing calcium carbonate to be deposited in the tank (the blue arrow).
What this zone implies is that both calcium and alkalinity are elevated, and that by removing calcium carbonate from the water, either through biotic deposition into coral skeletons or coralline algae, or through abiotic precipitation, as on heaters, the levels of each will drop in an appropriate ratio. More specifically, the tank parameters will move along a line parallel to the two lines bordering this zone, and directly into the red target zone (the blue arrow in Figure 2). If you are smack in the middle between these two lines, as in Figure 2, then you will continue to move in the middle of these two lines down into the target zone.
This movement can continue right out the bottom end of the target zone (into zone 2), of course, so once you reach the target zone, you’ll have to reinitiate normal calcium and alkalinity additions.
Corrections for Zone 2
Zone 2 is also an easy problem to correct, and is very common. In this case, both calcium and alkalinity are on the low side of normal. If you take any tank in the red target zone, and let corals and coralline algae grow (i.e., calcify), then you will move into zone 2. Just as above (Figure 2), you will move downward in a fashion such that you parallel the lines bordering the zone. That fact is why it is so common to have this problem: anyone not adding enough calcium and alkalinity to balance demand in the tank will likely enter this zone.

Figure 3. A graph showing how to correct values within zone 2 by supplementing with a balanced calcium and alkalinity additive system (the blue arrow).
To correct this situation back to the red target zone, one wants to add calcium and alkalinity in a balanced fashion. Fortunately, there are many ways to achieve this balance, such as with limewater (kalkwasser), calcium carbonate/carbon dioxide reactors, and the many balanced two-part calcium and alkalinity supplements (such as B-ionic, C-Balance, etc.). The more that you add of these, the further upward your correction will go (the blue arrow in Figure 3). Of course, adding too much will push you into zone 1, but if that happens you can just sit back and watch it drop back to the target zone. I’ll discuss balanced additives more at the end of the article.
Of course, you can add calcium and alkalinity that are not “balanced” (that is, not tied to one another in any specific ratio). For example, adding calcium chloride and sodium bicarbonate will work fine, but you must fine-tune exactly how much of each you add. Consequently, more careful monitoring of calcium and alkalinity levels is necessary if you go this route.
Remember that manufacturer recommendations are based on maintaining a tank, not in making substantial corrections. To make such corrections, you may need to add much more than is recommended. If you are adding a balanced additive (and the pH is not getting out of the range of about 7.9 to 8.5) then the worst that is likely to happen from overdosing (other than truly huge overdosing) is wasting some money and causing some extra precipitation of calcium carbonate on your heater and other parts of the tank. These balanced additives are discussed in more detail at the end of the article.
On the other hand, if you are using independent calcium and alkalinity additives, you must be very careful to not create an imbalance by adding too much of one relative to the other. Directions for deciding how much of these types of additives can be found below, but you should rely on frequent testing more than recommended amounts, to determine how much of these types of additives to put into the tank.
Finally, if you are adding large amount of calcium and alkalinity supplements, but just cannot maintain the desired values, you might want to measure the magnesium level in the water. Magnesium plays an important role in preventing the abiotic precipitation of calcium carbonate1, and if it is substantially depleted, you may be experiencing excessive amounts of calcium and alkalinity loss to this route. Magnesium gets the blame far more frequently, in my opinion, than it is likely responsible, but since it is easy to check with a test kit and easy to supplement if necessary, there’s no reason to not see if it is a problem. I’d advise aiming for a natural seawater level of about 1300 ppm.
Corrections for Zone 3
Zone 3 problems are a little harder to correct, and are fairly common. It is, in fact, the problem in the real question posed at the beginning of this article (it doesn’t say so there, but the alkalinity was 3.2 meq/L). This problem is typically caused by overdosing alkalinity RELATIVE to calcium, but does not necessarily imply that calcium is either too high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.
One more word about this zone before getting to solutions: Many tanks end up here because aquarists are trying to correct pH problems by adding “buffer.” In my opinion, one should not try to correct any pH problem by simply adding an alkalinity supplement. If you are low on alkalinity, it is a fine course of action to raise the alkalinity. But if alkalinity is OK, or even high, adding an alkalinity supplement to alter the pH may simply create a worse problem. Better solutions to pH problems are discussed in this recent article6.

Figure 4. A graph showing how to correct values within zone 3 by adding a calcium additive, such as calcium chloride (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the right hand edge of zone 3), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
I would advise correcting this problem by adding a calcium chloride supplement until you have moved into the target zone (or zones 1 or 2 that you can then treat as described above) as shown in Figure 4. Almost any brand of calcium chloride will do (Kent Turbo Calcium, Kent Liquid Calcium, ESV, etc.). Certain other calcium supplements may also be OK (such as just the calcium component of the two-part calcium and alkalinity additive systems), but you do not want to add any alkalinity. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the edge of the zone, while you want to move over to it, and cross it.
If calcium is less than 400 ppm, I’d suggest using this handy online calculator7 to determine how much dry calcium chloride is necessary to move back to the target zone. Note that it is a minimum estimate because it does not know how much alkalinity you have, so it cannot know if you are only raising calcium directly (which it calculates) or are also precipitating calcium carbonate (when alkalinity is high this will probably happen, but is typically not a problem other than that it uses up some of what you add).
If the calcium is above 400 ppm in this zone (unlikely, but it does happen), then you can safely either do nothing until it drops and you need to add more calcium, and treat it as suggested in the previous paragraph, or you can add some calcium immediately, move into zone 1, and then just let it drop on its own.
Corrections for Zone 4
Zone 4 problems are also a little harder to correct. It is typically caused by overdosing calcium RELATIVE to alkalinity, but does not necessarily imply that alkalinity is either to high or too low (though it is almost always too low). To correct problems in this zone, monitoring of calcium and alkalinity values during correction is especially important.

Figure 5. A graph showing how to correct values within zone 4 by adding an alkalinity additive, such as baking soda (the blue arrow).
If this problem is extreme (i.e., you are far from the line at the left hand edge of zone 4), then water changes may be the best way to correct to the problem. In most cases, however, water changes aren’t necessary.
If alkalinity were less than 4 meq/L (11 dKH; the most common situation in zone 4; shown in Figure 5), I would advise correcting this problem by adding an alkalinity supplement until you have moved into the target zone (or zone 1). For systems with a pH of 8.2 or above, baking soda (sodium bicarbonate) is a good choice. For systems with a pH below 8.2, washing soda (sodium carbonate) is a good choice (though use some baking soda too if the correction is a large one and the pH gets too high; that is, above pH 8.5 or so).
In gauging how much to add, here are some rough guidelines:
Baking Soda To raise 50 gallons of tank water by 1 meq/L will require about 16 grams of baking soda (sodium bicarbonate; sodium hydrogencarbonate). Since a level teaspoon of baking soda weighs just under 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.4 meq/L (~1 dKH). Washing Soda To raise 50 gallons of tank water by 1 meq/L will require 10 grams of washing soda (sodium carbonate). Since a level teaspoon of washing soda weighs just over 6 grams, then 1 teaspoon will raise the alkalinity in that 50 gallons by ~0.6 meq/L (~1.7 dKH).
One special note about washing soda: Apparently some Canadian brands of washing soda contain surfactants. VIP brand, in particular, contains them and a reef keeper using them on my advice turned his tank into a bubble bath. On questioning, the manufacturer did indicate that a surfactant is present. The same reef keeper says the local Arm & Hammer brand in Canada smells strongly of perfume. I’d avoid perfumed brands, if possible. My Arm & Hammer Super Washing Soda purchased in the US apparently contains no significant surfactants, and is not perfumed. Nevertheless, anyone using washing soda for the first time ought to put some in water and stir it around to see if soapy bubbles form. If so, I’d suggest finding another brand.
Many commercial alkalinity supplements will also be fine for this purpose, as long as no significant calcium is added. In general, I don’t prefer those that contain substantial borate. The alkalinity component of the two-part calcium and alkalinity additive systems would be OK. You CANNOT use limewater or a calcium carbonate/carbon dioxide reactor to correct this problem. Any of the balanced calcium and alkalinity additive systems will move you parallel to the line at the left edge of the zone, while you want to move over to it, and cross it.
If alkalinity is more than 4 meq/L (11 dKH; an uncommon situation), then you can safely either do nothing until it drops and you need to add more alkalinity, and treat it as suggested in the previous two paragraphs, or you can add some alkalinity immediately, move into zone 1, and then just let it drop on its own.
Last edited:











