- Joined
- Oct 6, 2015
- Messages
- 899
- Reaction score
- 1,269
I do not think algue could be the cause. When algue growe they consume CO2 and that Will not affect alk. Some algue consume HCO3 but also there it is not an effect on alk as to consume one HCO3 it is also consume one H.Ok... back to square one then! lol
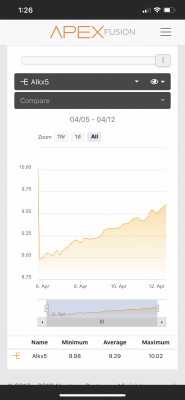
I'm going to try another bottle of reagent but I wouldn't expect an issue with the reagent to gradually give a higher reading each day. Could the algae die-off be providing something that could influence this?
Opposite, when algue die it releases organic carbon as the algue have fixed the inorganic carbon to organic in the photosyntes. Thus organic carbon is released in some way and that could maybe by bacteria be reoxidized to CO2 and again, CO2 Will not affect alk.