Hello,
My tank has been stable for the past months with my MRC Calcium Reactor. Recently I am more interested in dosing trace elements and I am trying to understand how much calcium my tank is consuming in order to know how much trace elements I need to dose. I am planning to use the Red Sea Trace Colors and their instructions says that I need to dose 1 ml of each bottle per 10ml of calcium consumed (10ml of Red Sea Foundation Calcium). What I am trying to do is try to understand the calcium consumed in my tank with my calcium reactor and try to convert those numbers to simulate the Red Sea foundation dosing program with the calcium reactor.
To start, I used Randy's CaRx calculator in order to know how much alkalinity my tank is consuming. Here's the numbers that I came up with:
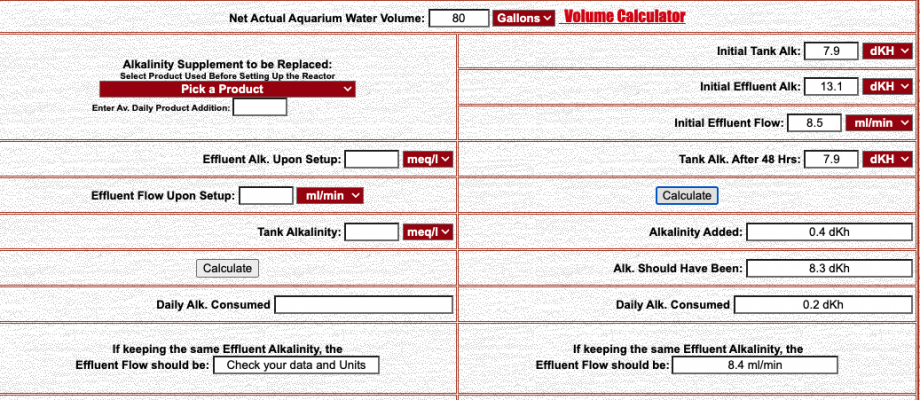
Basically what this is saying is that my tank is consuming 0.2 dKh every day. Now, how can I translate that 0.2 dKh consumption to Calcium consumption? That's the number I need in order to understand how much calcium I need to dose according to red sea and have my number for the trace elements program. Is there a way?
Thank you
My tank has been stable for the past months with my MRC Calcium Reactor. Recently I am more interested in dosing trace elements and I am trying to understand how much calcium my tank is consuming in order to know how much trace elements I need to dose. I am planning to use the Red Sea Trace Colors and their instructions says that I need to dose 1 ml of each bottle per 10ml of calcium consumed (10ml of Red Sea Foundation Calcium). What I am trying to do is try to understand the calcium consumed in my tank with my calcium reactor and try to convert those numbers to simulate the Red Sea foundation dosing program with the calcium reactor.
To start, I used Randy's CaRx calculator in order to know how much alkalinity my tank is consuming. Here's the numbers that I came up with:

Basically what this is saying is that my tank is consuming 0.2 dKh every day. Now, how can I translate that 0.2 dKh consumption to Calcium consumption? That's the number I need in order to understand how much calcium I need to dose according to red sea and have my number for the trace elements program. Is there a way?
Thank you
















