So I was wanting to get everyone's thoughts on the difrent types of products we use to dose our reef tank
I'm trying to see if there is a major difrence between cheap products like backing soda to control alk vs red sea alk vs a no name alk
The reason I want to know this is alot of people dose sodium bicarbonate to control alk
But the alk of a reef tank is more then just sodium bicarbonate
Alkalinity is not a chemical in water, but, rather, it is a property of water that is dependent on the presence of certain chemicals in the water, such as bicarbonates, carbonates, and hydroxides
Other common natural components that can contribute to alkalinity include borate, hydroxide, phosphate, silicate, dissolved ammonia, the conjugate bases of some organic acids (e.g., acetate), and sulfate.
So has anyone noticed a dfrence between just backing soda
And the ones that contain everything need for alk
Vs no names that we really don't know for sure every bottle has the same consintratiin
I would think borate is just as important as sodium bicarbonate and carbonate
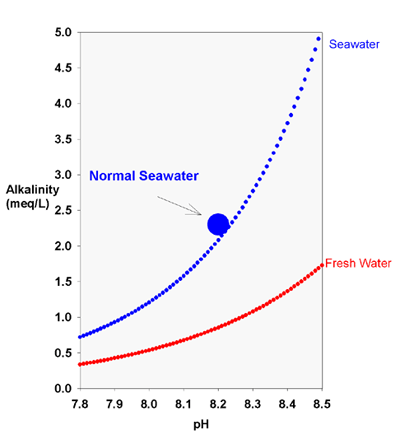
This lack of effective buffering at a normal tank pH is one of the reasons that some salt manufacturers (Seachem, as told to me by the late Leo Morin, and possibly to a lesser extent, Coralife) boost the borate concentrations in their salt mixes. Since the pKa of borate in seawater is about 8.6, its maximal b (pH 8.6) is not far from the range experienced by reef tanks. In normal seawater at pH 8, borate provides about half of the buffering against a small downward pH change, despite the fact that it provides less than 3% of the total alkalinity. In Seachem salt, where borate is about 10x natural levels, borate totally dominates the buffering in the pH range experienced by reef tanks.
See
 reefs.com
reefs.com
I'm trying to see if there is a major difrence between cheap products like backing soda to control alk vs red sea alk vs a no name alk
The reason I want to know this is alot of people dose sodium bicarbonate to control alk
But the alk of a reef tank is more then just sodium bicarbonate
Alkalinity is not a chemical in water, but, rather, it is a property of water that is dependent on the presence of certain chemicals in the water, such as bicarbonates, carbonates, and hydroxides
Other common natural components that can contribute to alkalinity include borate, hydroxide, phosphate, silicate, dissolved ammonia, the conjugate bases of some organic acids (e.g., acetate), and sulfate.
So has anyone noticed a dfrence between just backing soda
And the ones that contain everything need for alk
Vs no names that we really don't know for sure every bottle has the same consintratiin
I would think borate is just as important as sodium bicarbonate and carbonate
This lack of effective buffering at a normal tank pH is one of the reasons that some salt manufacturers (Seachem, as told to me by the late Leo Morin, and possibly to a lesser extent, Coralife) boost the borate concentrations in their salt mixes. Since the pKa of borate in seawater is about 8.6, its maximal b (pH 8.6) is not far from the range experienced by reef tanks. In normal seawater at pH 8, borate provides about half of the buffering against a small downward pH change, despite the fact that it provides less than 3% of the total alkalinity. In Seachem salt, where borate is about 10x natural levels, borate totally dominates the buffering in the pH range experienced by reef tanks.
See

Chemistry And The Aquarium: The Relationship Between Alkalinity And pH
Randy answers many questions in this complex relationship.
Last edited: