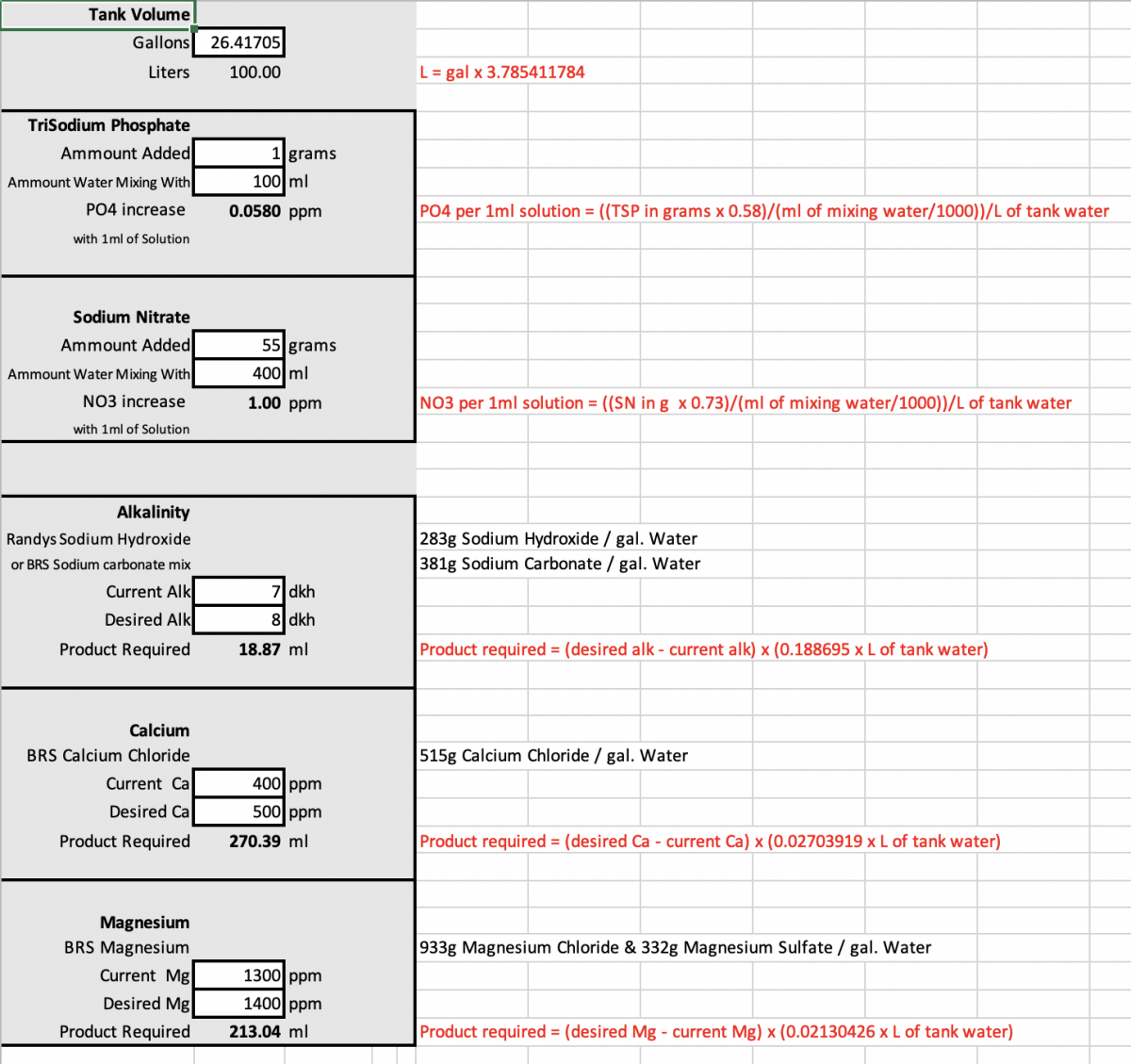
Hi, I wanted to have an excel sheet with my most used calculators on it. I reveresed engineered the formulas for Alk, Calcium, and magnesium from BRS so if there a a more proper formula please let me know.
For the TriSodium Phosphate and Sodium Chloride formulas I referenced @Randy Holmes-Farley posts on R2R, so hopefully I understood those correctly.
I wrote out the formulas in red on the right and worte the solution recipes above them in black.
How did I do and what would you fix?
I don't think the Alk recipe is perfect for randys solution but it is close enough to do the job. If someone could help my adjust the recipes to better match that would be great. I like haveing my alk recipes 1:1 so I can vary their dose based on my PH needs.

If anyone know the how to upload an excel file to R2R please let me know.
For the TriSodium Phosphate and Sodium Chloride formulas I referenced @Randy Holmes-Farley posts on R2R, so hopefully I understood those correctly.
I wrote out the formulas in red on the right and worte the solution recipes above them in black.
How did I do and what would you fix?
I don't think the Alk recipe is perfect for randys solution but it is close enough to do the job. If someone could help my adjust the recipes to better match that would be great. I like haveing my alk recipes 1:1 so I can vary their dose based on my PH needs.
If anyone know the how to upload an excel file to R2R please let me know.















