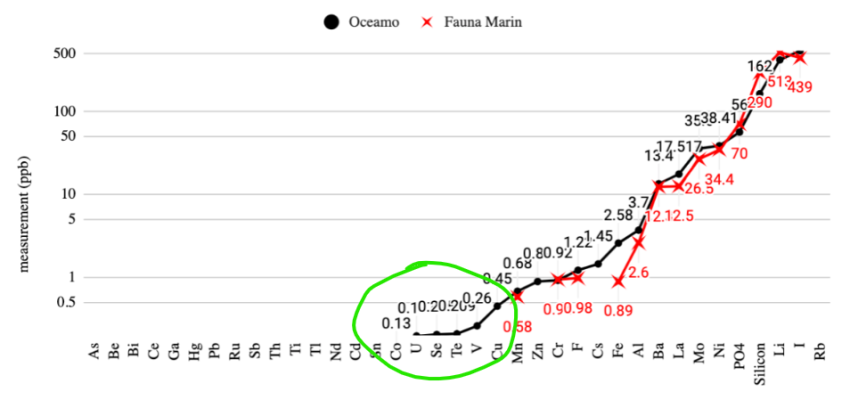
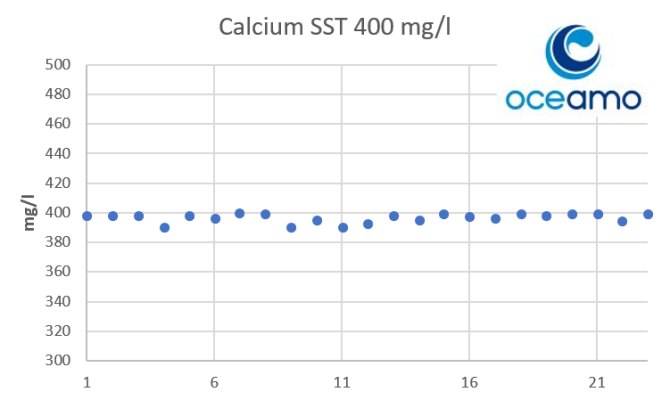
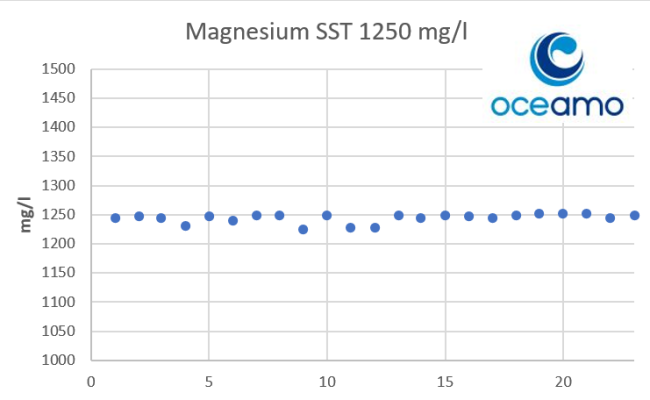
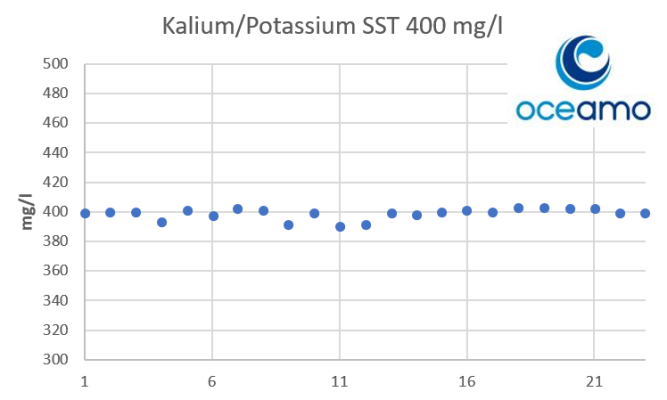
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
Certainly don't send samples to a bunch of ICP vendors. That way leads to madnessSkeptic or not, how do you decide on the amount of trace elements to dose or whether you have an elevated pollutant when the data can be rather uncertain?