- Joined
- Feb 12, 2019
- Messages
- 87
- Reaction score
- 35
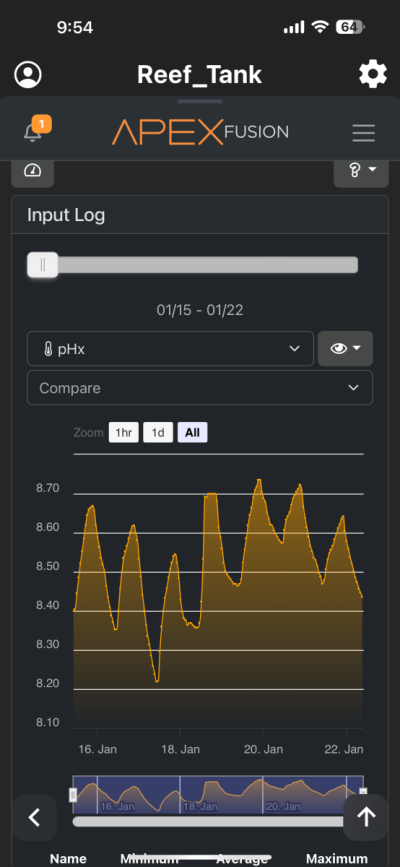
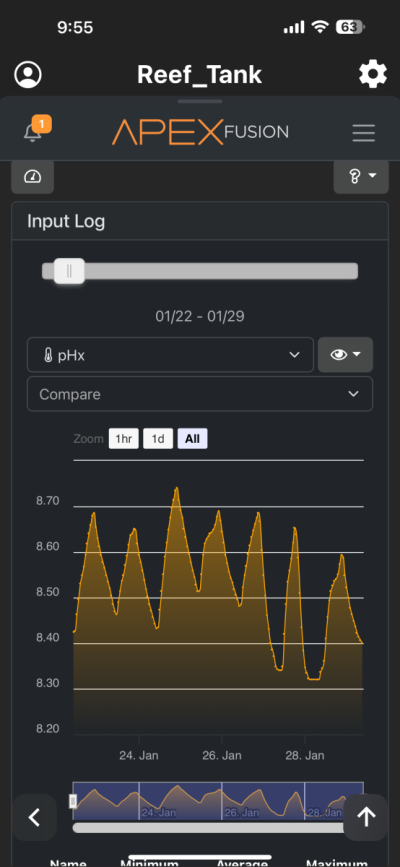
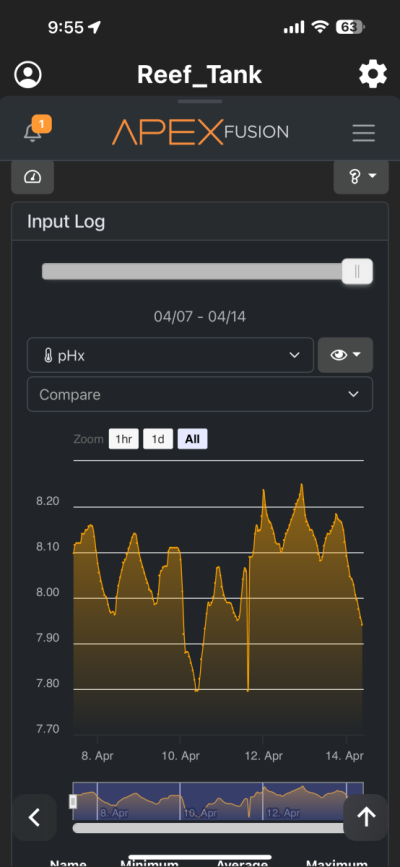
I have been chasing elevated pH for over a year now and I had great success. I was very happy with the results. However for whatever reason I am unable to achieve similar pH levels (8.3-8.6) I previously had despite keeping everything the same in my 150 gallon tank. I am using the following methods to increase pH (same as before) recirculating co2 scrubber, Kalkwasser through a stirrer 11L at night, recently added algae scrubber, keep house co2 400-600, dose sodium hydroxide to make up the difference between alk and calcium in Kalkwasser. As you can see from the charts I used to maintain fairly high pH with all my methods. But recently past few weeks, using the same methods I have been unable to raise it back to previous levels. Is there another cause? The only change that I know that happened was that I siphoned the sand since I spilled rowaphos when the media reactor lid came off into my tank which I don’t usually do. But I recalibrated both pH probes (I do this monthly but I double checked again), change co2 scrubber media, clean the skimmer just in case it wasn’t pulling as much air, added new fresh Kalkwasser to the stirrer but I am barely getting above 7.9 at times. Is there an underlying cause? Or are there other means I can increase the pH? I thought maybe disturbing my shallow sand bed (0.5-1 inch) may have increase organics which lowered the pH. But I have done large water changes and also refreshed the carbon as well as added ozone to the tank but I still cannot increase my tank pH. Any help would be appreciated. Thanks!