I’ll be interested to see your results of your test with Hannah checker. Please tag me.I think that is part of my surprise from this report. I haven't seen iron on my ICP OES reports, but this data is suggesting I can get an accurate reading from a Hanna checker. I agree it is utilized at high rates by coral and algae. Which makes it exciting I can monitor it more continuously with a home kit.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
HOBBY GRADE TEST KITS CAN OUTPERFORM ICP MEASUREMENTS…REALLY??
- Thread starter Rick Mathew
- Start date
- Tagged users None
- Joined
- Sep 9, 2018
- Messages
- 1,499
- Reaction score
- 1,129
Which labs used in this study is this comment aimed at, specifically?I can’t speak for KStatefan, but using less experienced labs or newly opened labs with unqualified staff and poor technique/ environment…
I contest that most folks in this hobby have no need to know the values of a majority of the elements given in an ICP analysis.....My argument is that most folks will be waaaaaay better off testing 40+ elements...
Sure will. I will order one and test on multiple systems. I will also do different times of day on my home system. I think that will be interesting. I'll let you knowI’ll be interested to see your results of your test with Hannah checker. Please tag me.
- Joined
- May 22, 2016
- Messages
- 6,597
- Reaction score
- 10,186
I am always confused with P/PO4:
I am really not sure what is compared here, I guess I just don’t understand P.
My understanding is Hanna checker regardless of type only measure Ortho-Phosphate PO4.
ICP measures P (element) then provides calculation for total PO4 (made up of all types and Ortho-phosphate is one).
Since the two methods measure different things what do the results mean?
I believe P/PO4total/PO4 Ortho etc… is critical for a reef tank yet I find it confusing on what is measured and what to do.
What was spiked was Phosphate to an amount of +20 ppb Phosphorus. Then hanna chemical tests measured the phosphate and found the majority of what was spiked, measured PO4, and results reported as P.
ICP measures PO4, and any other form of P, report it all as P as well - but they generally found less than the chemical tests did - even though ICP could in theory measure more forms. This means that there is likely loss of PO4 in the sample.
Note that unlike the other ICP vendors, Oceamo does a chemical PO4 test so they are also measuring PO4, like the hobby kit. They also use a dedicated stabilized sample for PO4. In this case the effort pays off and the result is closer to the amount spiked than other vendors and very close to what we the hobbyists measure (generally within our error bars).
Phosphorus is important and quite a challenge to measure well. Turns out it's hard to do much better than a hanna checker.
This is incredible work, and very useful. Thank you !
The day the hobby starts preaching a monthly $85 ICP test is required for a successful tank is the day we won’t have to worry about the hobby any longer. It will be gone.
Yeah, but that’s not the argument I’m making. My argument is that it’s just “smart reefing” to look at all the elements and actually see where they are. Do you not agree with that?
You tested multiple salt brands. The whole topic of this thread is about what? Test results! Why? Because they are extremely important to make smart dosing decisions that do what? Keep our water chemistry at a higher level, and prevent overdosing.
I get it though, not everybody can afford it. I can barely afford it! I’ve been selling Fragz to get ICP-MS, because I see the importance of that analysis, and results in my system. Many potential risks are minimized or eliminated, and the chemistry stays strong which promotes stability, color, and growth.
Most people aren’t arguing that you can’t have a successful reef without ICP testing. Sure you can. I just don’t believe most reefers will have the same stability, color, or growth speed, but that’s just my opinion. Let’s face it, most people in the hobby are 1-3 year reefers.
I don’t want to name names, but if the results are accurate, you can pretty much see who’s on top and who’s on bottom. This was not a surprise for me.Which labs used in this study is this comment aimed at, specifically?
I contest that most folks in this hobby have no need to know the values of a majority of the elements given in an ICP analysis.
You are correct that most reefers don’t need to know the values, because the tools that most companies have do it all for you.
As long as you can enter the values correctly It’ll tell you exactly what to do. You do need to understand basic math, but that’s about it.
I don’t think that‘s how it works.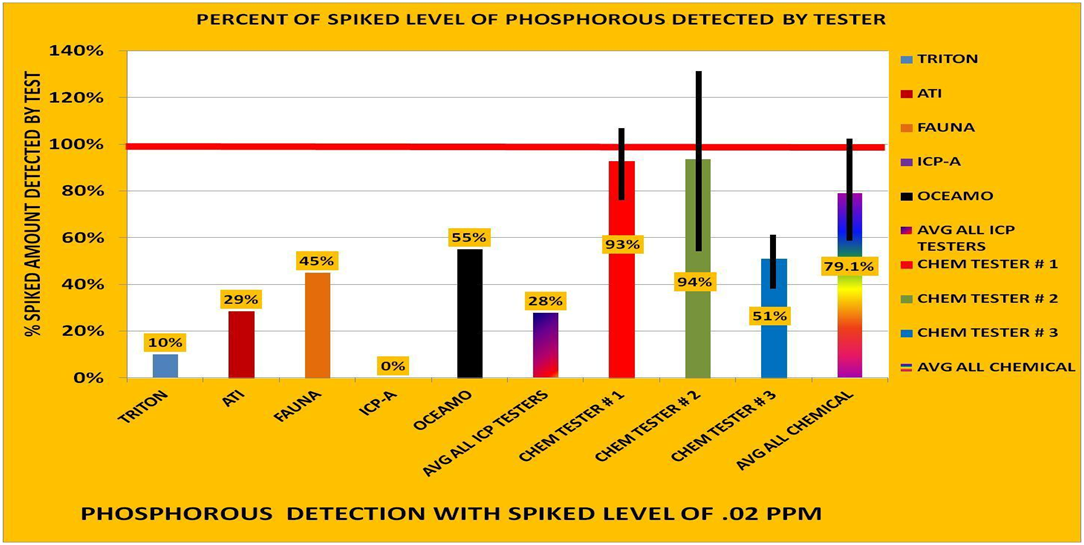
What was spiked was Phosphate to an amount of +20 ppb Phosphorus. Then hanna chemical tests measured the phosphate and found the majority of what was spiked, measured PO4, and results reported as P.
ICP measures PO4, and any other form of P, report it all as P as well - but they generally found less than the chemical tests did - even though ICP could in theory measure more forms. This means that there is likely loss of PO4 in the sample.
Note that unlike the other ICP vendors, Oceamo does a chemical PO4 test so they are also measuring PO4, like the hobby kit. They also use a dedicated stabilized sample for PO4. In this case the effort pays off and the result is closer to the amount spiked than other vendors and very close to what we the hobbyists measure (generally within our error bars).
Phosphorus is important and quite a challenge to measure well. Turns out it's hard to do much better than a hanna checker.
I suspect that what was added is some form of Ortho-Phosphate PO4 that Hanna can pick up. Hanna checkers only measure Ortho PO4, they do not measure P nor organic PO4 or poly phosphate etc… The checker performs calculation based on this to estimate P, I suspect based on the Ortho PO4.
ICP on the other hand measure P (element P and all of it) and generally reports is as total PO4.
Some companies perform titration test to provide Ortho PO4 so you get a ratio. Here is a sample:
Total PO4 (ICP) does not equal Ortho PO4 (Hanna). Maybe under some ideal conditions. Again Hanna P is not equal to ICP P, different methods, different calculations.
So my issue:
1) What is being compared? From my point of view they counted 5 apples (Hanna), 5 oranges (ICP). The conclusion is that Hanna is accurate at measuring 5 apples. Seems odd to me, maybe oranges are important!!!
2) If indeed OrthoPO4 was added and the sample only had OrthoPO4, I know from experience this form likes to bind to everything so after 3 days in transit there should be a drop. ICP did indeed find less P (expected result) yet Hanna did not (questionable result or interpretation).
In statistics they say give me data, tell me what you want me to find and I will prove it... I feel that some of that was done here.
Review and comments by some of the ICP companies might have been beneficial. Especially since they are being crucified for the greater good.
Also don’t get me started on I and RedSea test kit. That right there is a red flag for me. I would really like for some experts (neutral experts) to reviewed the method and conclusion … I used the test and always had perfect I value, test is in garbage now…
Regardless great work, I just question some of the method and conclusions. Seems one sided.
Please feel free to correct me, chemistry is not my field.
But I am learning and things don’t compute hence my comments.
ICP measures PO4, and any other form of P, report it all as P as well - but they generally found less than the chemical tests did - even though ICP could in theory measure more forms. This means that there is likely loss of PO4 in the sample.
It’s kinda a bummer that most labs calculate the PO4 values from the measured P instead of directly measuring both. I never really liked that, but over the years it does seem to be very close.
If the lab is not filtering the sample or using a nutrient stabilizer to reduce or limit microbial activity, I’m sure the PO4 will be slightly lower as you did find with your experiment.
In all fairness I did see a little better accuracy with filtered samples from OCEAMO vs non-filtered samples from ATI, but honestly not that much. ATI was still very close to my Hanna Phosphorus ULR results. I believe in another year or two that all labs will be filtering and using stabilizer. Most EU labs are doing it already.
Last edited:
So my issue:
1) What is being compared? From my point of view they counted 5 apples (Hanna), 5 oranges (ICP). The conclusion is that Hanna is accurate at measuring 5 apples. Seems odd to me, maybe oranges are important!!!
Yes, you can’t compare the two accurately because ICP is detecting the Atom P which may come from organic forms as well as inorganic phosphate. Our Hanna’s are only detecting inorganic phosphate. I know these guys do understand that so I’m a little confused myself.
This may help…Dr. Balling had to educate me a while back because I become confused also. I saved these important words:
————————————————————-
Hans-Werner Balling:
I think the phosphorus - phosphate confusion is kind of funny, I'm sorry!
Finally it is quite simple (and you can even find it in Wikipedia): Nearly all phosphorus in biochemistry and in the environment is phosphate, no matter whether organic or inorganic phosphate, all these phosphates are in the oxidation state +5.
Only a small proportion of phosphorus in biochemistry is phosphonates. However, at least one scientific article says, corals can also make use of phosphonates. This only to add some confusion.
Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate.
There are three kinds of phosphates, orthophosphate, inorganic polyphosphates (or pyrophosphates) and organic phosphates. While wet chemical tests without a prior step only find orthophosphate completely, ICP-analysis finds all kinds of phosphate compounds as phosphorus.
Polyphosphates and organic phosphates both differ from orthophosphate by having high-energy phosphate bonds. For wet chemical analysis these bonds have to be hydrolysed by a digestions step prior to normal orthophosphate analysis.
Inorganic polyphosphates are for example storage phosphates of algae and bacteria, but where also used as water softeners in washing detergents.
In oligotrophic reefs the polyphosphates and organic phosphates may be a significant proportion of total phosphate and exceed the orthophosphate concentration. Corals and many (most?) other organisms can make use of organic phosphates and inorganic polyphosphates by excreting enzymes, the alkaline phosphatases, that break down these phosphates to orthophosphate ready for uptake.
To make a long story short, it are all phosphates and corals can use them all for phosphate, but our test kits and photometers only find orthophosphate and in this way may underestimate the phosphate concentration, especially if phosphate concentrations are low and a high proportion of the phosphates are inorganic polyphosphates and organic phosphates of algae and bacteria.
————————————————————-
Hans-Werner Balling:
I think the phosphorus - phosphate confusion is kind of funny, I'm sorry!
Finally it is quite simple (and you can even find it in Wikipedia): Nearly all phosphorus in biochemistry and in the environment is phosphate, no matter whether organic or inorganic phosphate, all these phosphates are in the oxidation state +5.
Only a small proportion of phosphorus in biochemistry is phosphonates. However, at least one scientific article says, corals can also make use of phosphonates. This only to add some confusion.
Elemental phosphorus is virtually non existent, just like sodium as metal. Both are too reactive to exist as element or metal respectively. So we don't need to discuss phosphorus, for us all phosphorus is simply phosphate.
There are three kinds of phosphates, orthophosphate, inorganic polyphosphates (or pyrophosphates) and organic phosphates. While wet chemical tests without a prior step only find orthophosphate completely, ICP-analysis finds all kinds of phosphate compounds as phosphorus.
Polyphosphates and organic phosphates both differ from orthophosphate by having high-energy phosphate bonds. For wet chemical analysis these bonds have to be hydrolysed by a digestions step prior to normal orthophosphate analysis.
Inorganic polyphosphates are for example storage phosphates of algae and bacteria, but where also used as water softeners in washing detergents.
In oligotrophic reefs the polyphosphates and organic phosphates may be a significant proportion of total phosphate and exceed the orthophosphate concentration. Corals and many (most?) other organisms can make use of organic phosphates and inorganic polyphosphates by excreting enzymes, the alkaline phosphatases, that break down these phosphates to orthophosphate ready for uptake.
To make a long story short, it are all phosphates and corals can use them all for phosphate, but our test kits and photometers only find orthophosphate and in this way may underestimate the phosphate concentration, especially if phosphate concentrations are low and a high proportion of the phosphates are inorganic polyphosphates and organic phosphates of algae and bacteria.
If you are confused, I am lost… Where is Randy or Hans-Werner so they can provide 5 more papers to read up on P. That should keep me busy for another month…Yes, you can’t compare the two accurately because ICP is detecting the Atom P which may come from organic forms as well as inorganic phosphate. Our Hanna’s are only detecting inorganic phosphate. I know these guys do understand that so I’m a little confused myself.
If you are confused, I am lost… Where is Randy or Hans-Werner so they can provide 5 more papers to read up on P. That should keep me busy for another month…
I listen to Randy very carefully too. Let’s let his words speak now. I save everything:
————————————————————-
Randy Farley:
You are not dosing phosphorus. It is not stable in water and burns when wet. It's a weapon of war (e.g., white phosphorus grenades).
The Hanna is detecting inorganic phosphate, regardless of how it chooses to report the data (some are in ppb phosphorus, some in ppm phosphate).
ICP detects only the atom P, and it may come from organic forms of phosphate (such as DNA, phospholipids, etc.) as well as inorganic phosphate.
———————————————————
Randy Farley:
ICP detects atoms like phosphorus, regardless if the chemical form.
Phosphate is one form phosphorus can take, consisting of oxygen, phosphorus and, in most situations in seawater, hydrogen.
In most scenarios in a reef tank, inorganic phosphate is higher in concentration than other forms of phosphorus, such as organic phosphates.
you can dose organic or inorganic phosphate, if you want to dose, but must folks elect to dose inorganic phosphate, such as sodium phosphate.
How about Christoph. We should all have a good understanding now. HaHa!
———————————————————-
Christoph:
Hello everybody!
We do measure total phosphorus (via ICP-OES or ICP-MS, depending on which method the customer chooses) and also orthophosphate (PO4) using a photometric method based on the molybdenum blue/ascorbic acid method. We are using a Shimadzu Lab photometer with 4 cm optical path to have low detection limits. We do not calculate a phosphate value from the ICP data.
Phosphate is not very sensitive on ion chromatography (since it has a low specific conductivity), thus IC is not very useful for detection of phosphate in reef tanks. Photometry is the superior method in this case.
In case of higher phosphate levels total phosphorus and orthophosphate often agree very well - so most of the phosphorus in the sample is actually phosphate. This is not always the case, especially at lower nutrient levels, where a significant proportion of total phosphorus can be something else (might be oligophosphates or DNA or other phosphorus containing molecules).
We are using an orthophosphate based salt for phosphorus dosing, and i would be surprised if other products/brands would use other phosphorus sources. In my opinion it is important to dose phosphate spread out over several dosings per day, to achieve a constant availability. Otherwise this important nutrient might be depleted fast - either by consumption/metabolism, or by adsorption onto surfaces. Cycling between "available state" and "limitation" is imo stressful for corals, and should thus be avoided.
Best regards,
Christoph
———————————————————-
Christoph:
Hello everybody!
We do measure total phosphorus (via ICP-OES or ICP-MS, depending on which method the customer chooses) and also orthophosphate (PO4) using a photometric method based on the molybdenum blue/ascorbic acid method. We are using a Shimadzu Lab photometer with 4 cm optical path to have low detection limits. We do not calculate a phosphate value from the ICP data.
Phosphate is not very sensitive on ion chromatography (since it has a low specific conductivity), thus IC is not very useful for detection of phosphate in reef tanks. Photometry is the superior method in this case.
In case of higher phosphate levels total phosphorus and orthophosphate often agree very well - so most of the phosphorus in the sample is actually phosphate. This is not always the case, especially at lower nutrient levels, where a significant proportion of total phosphorus can be something else (might be oligophosphates or DNA or other phosphorus containing molecules).
We are using an orthophosphate based salt for phosphorus dosing, and i would be surprised if other products/brands would use other phosphorus sources. In my opinion it is important to dose phosphate spread out over several dosings per day, to achieve a constant availability. Otherwise this important nutrient might be depleted fast - either by consumption/metabolism, or by adsorption onto surfaces. Cycling between "available state" and "limitation" is imo stressful for corals, and should thus be avoided.
Best regards,
Christoph
That is interesting, now can we get a value what is considered higher and did this experiment get to that threshold!!!In case of higher phosphate levels total phosphorus and orthophosphate often agree very well - so most of the phosphorus in the sample is actually phosphate. This is not always the case, especially at lower nutrient levels, where a significant proportion of total phosphorus can be something else (might be oligophosphates or DNA or other phosphorus containing molecules).
If that is the case perhaps Hanna is accurate at this higher level???
Other value maybe not!!!
Yeah, but that’s not the argument I’m making. My argument is that it’s just “smart reefing” to look at all the elements and actually see where they are. Do you not agree with that?
You tested multiple salt brands. The whole topic of this thread is about what? Test results! Why? Because they are extremely important to make smart dosing decisions that do what? Keep our water chemistry at a higher level, and prevent overdosing.
I get it though, not everybody can afford it. I can barely afford it! I’ve been selling Fragz to get ICP-MS, because I see the importance of that analysis, and results in my system. Many potential risks are minimized or eliminated, and the chemistry stays strong which promotes stability, color, and growth.
Most people aren’t arguing that you can’t have a successful reef without ICP testing. Sure you can. I just don’t believe most reefers will have the same stability, color, or growth speed, but that’s just my opinion. Let’s face it, most people in the hobby are 1-3 year reefers.
There’s an old saying I will paraphrase:
“The opinions of 10,000 men are of little value if none of them are educated on the topic.”
My own spin:
“The values of 40+ elements are of little value if none of them are accurate.”
Of course there is value in knowing our water chemistry. There is great value if those numbers are accurate and repeatable. I am not arguing against any of that.
My argument is that most ICP tests in this hobby are not accurate. Especially when none of the current companies provide any data to show how accurate their machine and tests are. Yes, Cristoph gave some great data but that is 1 out of a dozen and he doesn’t give that data on every report.
Because of my salt testing, I have been able to talk to universities that perform ICP testing that have no monetary involvement in this hobby. It is clear to me that none of them would promote the results we are given. Multiple of them told me it’s a minimum of $500 for them to run a test with any certainty.
I am not condemning ICP testing or any company providing it. I do think there is value in it. As we see more and more done, we will learn of the better companies and which elements seem to test accurately and reliably. But it is clear that some companies and elements do not test well. I am not educated in the least to go into depth on that. I can simply see patterns and make my best guess.
The 1% of reefers that want to go down the rabbit hole of ICP testing, dosing, testing, dosing, repeat repeat repeat…should. Trailblazers make new paths for us to follow. Sometimes those paths end falling off a cliff
The average reefer has no business ICP testing IMO. I see it almost daily in the AskBRS group. Normal everyday hobbyist was told to ICP test so they did and now have 50+ values to try and work out. It’s just assumed that ICP is superior and we must follow what it says. It’s only because of my experience in reading 100+ tests that I can tell things are or aren’t lining up. I can also see the company they used likely is or isn’t credible. But they are now lost chasing a ghost they will never catch.
The fact is they should have never sent an ICP to begin with. Educating the masses on the intricacies of ICP results is futile.
There’s an old saying I will paraphrase:
“The opinions of 10,000 men are of little value if none of them are educated on the topic.”
My own spin:
“The values of 40+ elements are of little value if none of them are accurate.”
Of course there is value in knowing our water chemistry. There is great value if those numbers are accurate and repeatable. I am not arguing against any of that.
My argument is that most ICP tests in this hobby are not accurate. Especially when none of the current companies provide any data to show how accurate their machine and tests are. Yes, Cristoph gave some great data but that is 1 out of a dozen and he doesn’t give that data on every report.
Because of my salt testing, I have been able to talk to universities that perform ICP testing that have no monetary involvement in this hobby. It is clear to me that none of them would promote the results we are given. Multiple of them told me it’s a minimum of $500 for them to run a test with any certainty.
I am not condemning ICP testing or any company providing it. I do think there is value in it. As we see more and more done, we will learn of the better companies and which elements seem to test accurately and reliably. But it is clear that some companies and elements do not test well. I am not educated in the least to go into depth on that. I can simply see patterns and make my best guess.
The 1% of reefers that want to go down the rabbit hole of ICP testing, dosing, testing, dosing, repeat repeat repeat…should. Trailblazers make new paths for us to follow. Sometimes those paths end falling off a cliff
The average reefer has no business ICP testing IMO. I see it almost daily in the AskBRS group. Normal everyday hobbyist was told to ICP test so they did and now have 50+ values to try and work out. It’s just assumed that ICP is superior and we must follow what it says. It’s only because of my experience in reading 100+ tests that I can tell things are or aren’t lining up. I can also see the company they used likely is or isn’t credible. But they are now lost chasing a ghost they will never catch.
The fact is they should have never sent an ICP to begin with. Educating the masses on the intricacies of ICP results is futile.
I tend to lean towards this too. I think on paper icp looks good, but the implementation of it is where it falls apart (sometimes). What really bothers me is that it is a crucial part for those dosing individual elements based on test results, and expecting to supplement in real time what your reef is depleting. I view that as equivalent to throwing darts while blindfolded. I still don't understand the logistics of dosing based on test results from weeks ago.
I know this thread isn't supposed to be debating the validity of icp, but rather looking at how hobby grade kits can outperform icp.
There’s an old saying I will paraphrase:
“The opinions of 10,000 men are of little value if none of them are educated on the topic.”
My own spin:
“The values of 40+ elements are of little value if none of them are accurate.”
Of course there is value in knowing our water chemistry. There is great value if those numbers are accurate and repeatable. I am not arguing against any of that.
My argument is that most ICP tests in this hobby are not accurate. Especially when none of the current companies provide any data to show how accurate their machine and tests are. Yes, Cristoph gave some great data but that is 1 out of a dozen and he doesn’t give that data on every report.
Because of my salt testing, I have been able to talk to universities that perform ICP testing that have no monetary involvement in this hobby. It is clear to me that none of them would promote the results we are given. Multiple of them told me it’s a minimum of $500 for them to run a test with any certainty.
I am not condemning ICP testing or any company providing it. I do think there is value in it. As we see more and more done, we will learn of the better companies and which elements seem to test accurately and reliably. But it is clear that some companies and elements do not test well. I am not educated in the least to go into depth on that. I can simply see patterns and make my best guess.
The 1% of reefers that want to go down the rabbit hole of ICP testing, dosing, testing, dosing, repeat repeat repeat…should. Trailblazers make new paths for us to follow. Sometimes those paths end falling off a cliff
The average reefer has no business ICP testing IMO. I see it almost daily in the AskBRS group. Normal everyday hobbyist was told to ICP test so they did and now have 50+ values to try and work out. It’s just assumed that ICP is superior and we must follow what it says. It’s only because of my experience in reading 100+ tests that I can tell things are or aren’t lining up. I can also see the company they used likely is or isn’t credible. But they are now lost chasing a ghost they will never catch.
The fact is they should have never sent an ICP to begin with. Educating the masses on the intricacies of ICP results is futile.
Let’s just post our ICP results and find out who’s closer to target on all values so we can let everybody decide. If you don’t have one then I can pay for one, and we can collect the samples on the same day and send them in. Or I have one now to share if you do.
Going off your logic…If ICP is not accurate, there should be no way my results would be closer. There should be no way my trace metals would be tighter in the “ultra trace” range where we’re targeting between 0.2 - 1 ug/L. You should also have less pollutants if ICP isn’t accurate. How confident are you with what you’re saying?
You know this is all in fun as we’ve both been around a long time. So if I win, you have to join our Group and send ICP-MS monthly for 6 months, and make all the corrections, and give your honest opinions afterwards on color, growth, and stability. If you win, I’ll remove my Metal Haldies and Calcium Reactor from my system. Not!
I’ll also extend the same challenge to anybody else who thinks ICP is not accurate.
- Joined
- May 22, 2016
- Messages
- 6,597
- Reaction score
- 10,186
2) is indeed basically what happened....2) If indeed OrthoPO4 was added and the sample only had OrthoPO4, I know from experience this form likes to bind to everything so after 3 days in transit there should be a drop. ICP did indeed find less P (expected result) yet Hanna did not (questionable result or interpretation).
We did spike PO4 because that is common, is the dominant form, and what can be measured easily by everyone - ICP and photometric chemistry.
The amount spiked was +20ppb.
The average of spike measured by the three hobby hanna testers was +17.4ppb and then after we retested our stored samples a week later the spike was measured at +14.4ppb P. So there was some detectable loss under our storage conditions, but it is inside our error bars thus maybe not significant. So we average it and increase the size of our testing error bars in the chart.
Why did we lose a small amount, but ICP vendors lost more - (except with stabilizer)? Our samples also got shipped across the country and were in transit for days too.
The likeliest reason I can point to is that our samples were kept in 500mL bottles, and ICP samples ship in the small tubes.
(Repeat this exact same discussion for Iron, BTW. We found some loss over 1 week storage - ICP vendors found almost total loss.)
Also ICP is simply has a more difficult time with P than a good photometric chem test. It's why oceamo does what they do, and See Sanjay's data for a second opinion.
P by ICP machine has wide disagreement.....
PO4 by colorimetric chemistry is much tighter...
Similar threads
- Replies
- 6
- Views
- 197



















