Very curious. Hope someone has some insight.
I recently had to treat a fish for intestinal worms. I decided on a 2-dose regimen of PraziPro and 2 weeks of Metroplex laced food. This meant 72 hrs no Skimmer / GAC, 48 hrs yes Skimmer / GAC, then another 72 hrs no skimmer /GAC.
1st 72 hrs of no skimmer / GAC and pH slowly came down.
48 hrs of back to normal brought my pH back to 8.35ish.
36 hrs into 2nd no skimmer / GAC pH has plummeted and I had to turn off Kalk dosing because Alk has climbed over 9.6dKH.
Red arrow indicates where the skimmer was turned off and green when it was turned back on. Skimmer has a recirculating CO2 scrubber (which is its primary function in my tank)
\
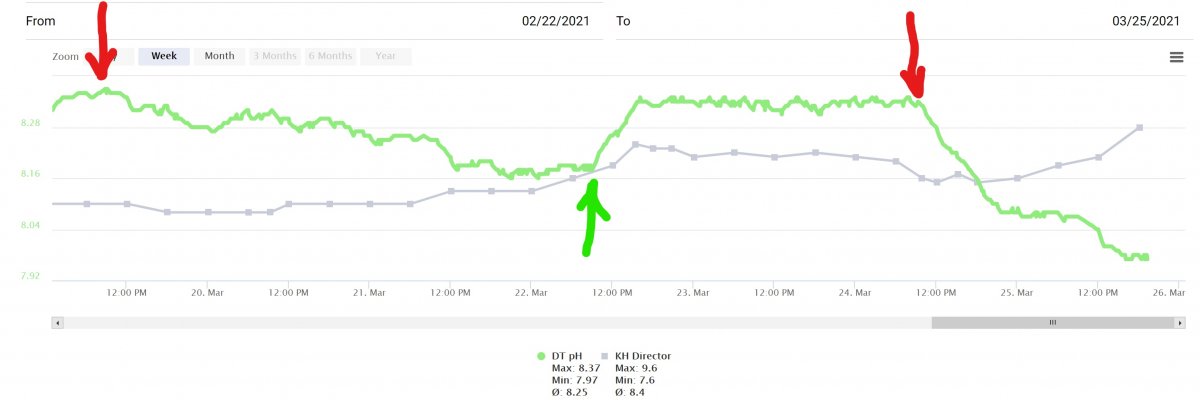
My question is why did the pH drop nice and slowly the 1st time and so quickly the 2nd time?
I recently had to treat a fish for intestinal worms. I decided on a 2-dose regimen of PraziPro and 2 weeks of Metroplex laced food. This meant 72 hrs no Skimmer / GAC, 48 hrs yes Skimmer / GAC, then another 72 hrs no skimmer /GAC.
1st 72 hrs of no skimmer / GAC and pH slowly came down.
48 hrs of back to normal brought my pH back to 8.35ish.
36 hrs into 2nd no skimmer / GAC pH has plummeted and I had to turn off Kalk dosing because Alk has climbed over 9.6dKH.
Red arrow indicates where the skimmer was turned off and green when it was turned back on. Skimmer has a recirculating CO2 scrubber (which is its primary function in my tank)
\
My question is why did the pH drop nice and slowly the 1st time and so quickly the 2nd time?














