- Joined
- Sep 21, 2018
- Messages
- 6,687
- Reaction score
- 7,178
A recent conversation with @taricha and @Lasse on the subject of cyanobacteria mats causing phosphate release from the substrate stimulated my interest in measuring the iron and phosphate content of aquarium substrate. I also looked at new Caribsea aragonite sand and the fines washed out with RO/DI. Conversations with @flampton, @brandon429 and @taricha helped shape the design of my experiments. The analysis involved dissolving the new aragonite sand and then adjusting the pH to about 5. Blanks were run to measure any Fe and PO4 in the hydrochloric acid and sodium hydroxide used in the experiments. Measurements were made with Hanna PO4 and Fe Checkers. Data is summarized in the plot below.
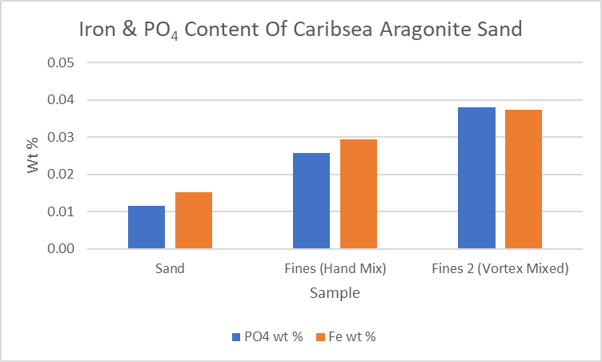
The fines, or dust, removed by rinsing the sand with water contain a higher concentration of PO4 and iron than the pre-washed sand (“Sand”). The fines from the more vigorously mixed sand-water mixture (“Fines 2 (Vortex Mixed)”) seem to be somewhat more enriched. Because only a tiny fraction of the sand is made up of fines, their removal is inconsequential to the PO4 and iron content of the sand. Given that the error bars on these measurements are at least 10%, the ratio of PO4 to iron is probably near 1:1.
What do these measurement mean? They could be important if the PO4 and iron are accessible to microorganisms, something that needs looking into. Also, there seems to be a small amount of organic matter present in Caribsea aragonite sand that supports bacterial growth.
The fines, or dust, removed by rinsing the sand with water contain a higher concentration of PO4 and iron than the pre-washed sand (“Sand”). The fines from the more vigorously mixed sand-water mixture (“Fines 2 (Vortex Mixed)”) seem to be somewhat more enriched. Because only a tiny fraction of the sand is made up of fines, their removal is inconsequential to the PO4 and iron content of the sand. Given that the error bars on these measurements are at least 10%, the ratio of PO4 to iron is probably near 1:1.
What do these measurement mean? They could be important if the PO4 and iron are accessible to microorganisms, something that needs looking into. Also, there seems to be a small amount of organic matter present in Caribsea aragonite sand that supports bacterial growth.
















