- Joined
- Sep 13, 2018
- Messages
- 360
- Reaction score
- 333
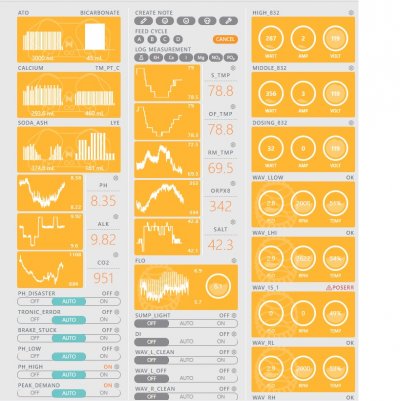
My system uses a ton of standard two part- 5dkh/day and rising. I'm looking to minimize mixing, refilling jugs and doser noise. I use roughly 45ml of bicarbonate, 375ml soda ash and 165ml lye per day. my alkatronic tests and I have a system setup through apex to fine tune alk while keeping pH high and stable as possible. The results have been truly incredible. Once a week I will test calcium, dose more if needed and adjust the schedule to match that week's alk usage. Currently I use double strength calcium solution(1,000g/gal) and ballpark my dose using this formula- (ml Bicarb/2)+ ml Soda ash + ml Lye and divide the whole number by 2. so 22.5+375+165=562.5 562/2= 281. This has worked well so far and making the weekly calculation just takes a quick look at my dos graphs. I'd like to apply this same basic principle while keeping most fluids at maximum saturation. Aside from bicarbonate, Soda ash seems to be the main limiting factor. Wikipedia lists solubility in water at a handful of temperatures and the best I can infer shows it has a solubility of ~245g/L at room temp. The solution strengths I'm proposing calls for 285g/L- supersaturated once cool. I mixed up a test liter and don't see any crystallization so I think this is OK. I'll have to consult Tropic marin about how much I can concentrate their part C. I have a feeling they'll suggest keeping it at 171g/gal as I have been doing; to match the standard strength alk solutions. I know Randy has sulfates in many of his formulations. Is this not a requirement since I'm using tropic marin part C to address ionic imbalance from part A/B?
Maximum concentration two part- Strengths listed in relation to RHF#1- Water volumes are rough starting points. Top up jugs to the 1 gallon mark after mixing.
5X Calcium
2500g CaCl2 Dihydrate
2650ml RODI
943.4g/L Max saturation= ~1120g/L @20C
0.5X Bicarbonate
300g NaHCO3
3600ml RODI
83g/L Max saturation= 96g/L
2.5X Soda Ash
955g Na2CO3
3500ml RODI
285g/L ***Max saturation @20C ~245g/L***
2.5X Lye
707g NaOH
3300ml RODI
~211g/L Max satuation= 1,000g/L
5X Magnesium
1306g MgCl2 hexahydrate
2600ml RODI
Max saturation= 2,350g/L- well under max
2.5X? Tropic Marin part C
428g Part C
~3500ml RODI
*Call and discuss precipitation with Lou @TM USA*
Maximum concentration two part- Strengths listed in relation to RHF#1- Water volumes are rough starting points. Top up jugs to the 1 gallon mark after mixing.
5X Calcium
2500g CaCl2 Dihydrate
2650ml RODI
943.4g/L Max saturation= ~1120g/L @20C
0.5X Bicarbonate
300g NaHCO3
3600ml RODI
83g/L Max saturation= 96g/L
2.5X Soda Ash
955g Na2CO3
3500ml RODI
285g/L ***Max saturation @20C ~245g/L***
2.5X Lye
707g NaOH
3300ml RODI
~211g/L Max satuation= 1,000g/L
5X Magnesium
1306g MgCl2 hexahydrate
2600ml RODI
Max saturation= 2,350g/L- well under max
2.5X? Tropic Marin part C
428g Part C
~3500ml RODI
*Call and discuss precipitation with Lou @TM USA*















