Nano 12g 'mixed reef' (SPS, LPS, Shrooms, Zoas) has been running continuously for 6-1/2 years:

No issues (good fish/coral health and color), but thought it might be interesting to see an element analysis.
12g Nano AIO (no sump) - 6-1/2 years old
50/50 Reef Crystals/Instant Ocean <- for the last year...previously MicroLift)
15%/wk water change
No mechanical or chemical filter media (live rock & live sand only)
Additives: Kalwasser, Kent Concentrated Iodine (3-4 drops/week) and ESV B-Ionic Magnesium
I'd like to see salinity and alkalinity tested and hopefully that's something that can be added in the future.
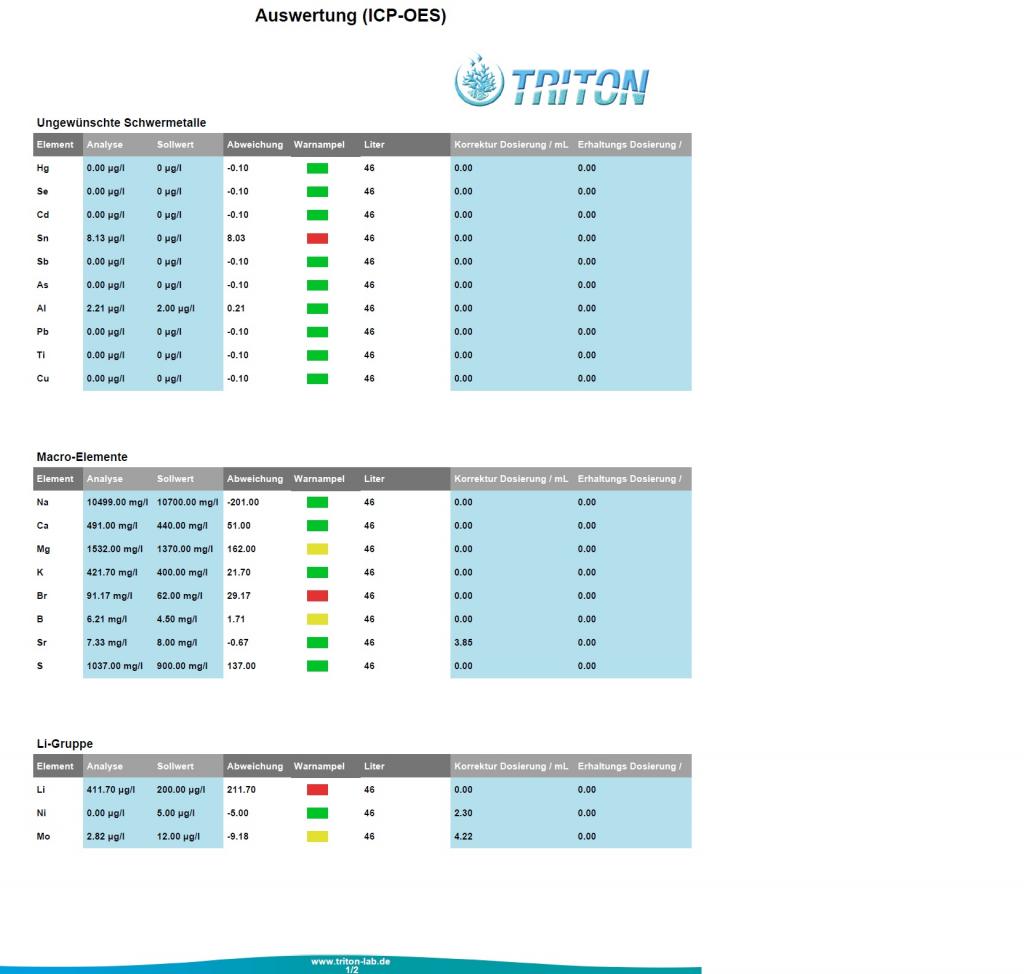
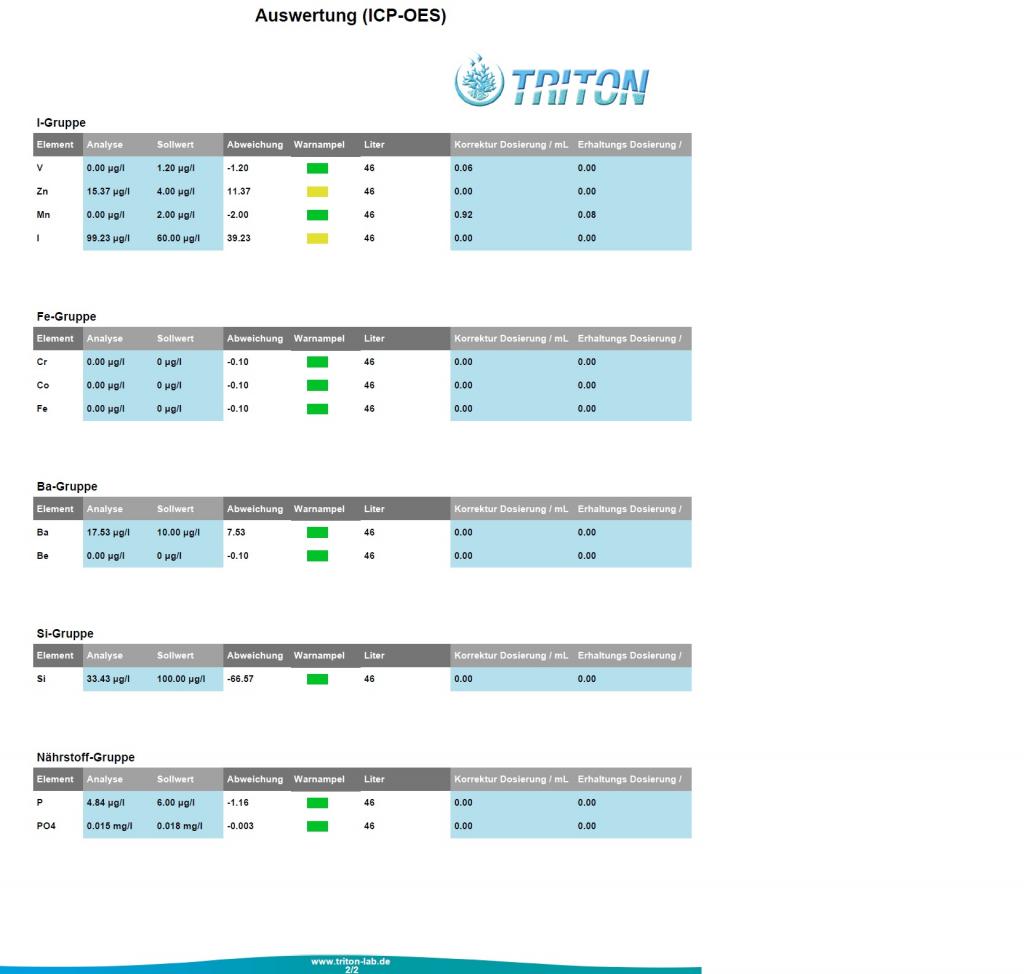

No issues (good fish/coral health and color), but thought it might be interesting to see an element analysis.
12g Nano AIO (no sump) - 6-1/2 years old
50/50 Reef Crystals/Instant Ocean <- for the last year...previously MicroLift)
15%/wk water change
No mechanical or chemical filter media (live rock & live sand only)
Additives: Kalwasser, Kent Concentrated Iodine (3-4 drops/week) and ESV B-Ionic Magnesium
I'd like to see salinity and alkalinity tested and hopefully that's something that can be added in the future.


Last edited:









