PART 4 "GETTING IT RIGHT" COLORIMETRIC INSTRUMENTAL TESTING METHODS (DIGITAL)
As we turn our attention to the use of digital testers (Colorimeters) such as the HANNA CHECKER, most of the same elements necessary for good precision and accuracy that exist in Visual Testing also exist in Digital Testing. Digital Testing methods have the same two steps as the Visual method: Evaluation (The Chemistry) and Assessment. Evaluation is the test procedure itself and Assessment is the determination of the results.
TESTING PROCEDURES (EVALUATION)
Most test kits consist of a series of reagents added to the sample according to a prescribed procedure. The procedure generally requires that the reagents be measured out by “drops”, ml or some type of measuring spoon. They are added to the sample being tested in prescribed order. There is usually a time element associated with the procedure…Mix for 1 min…wait for 30 seconds…etc. Part of the procedure using digital tests most often requires the measurement of a “Blank”. The Blank is the initial measurement that is taken in order to compensate for the “color” of the test sample itself (Water Color). After the last step in the procedure the sample is placed into the device for measurement. The device measures the light absorbed by the sample across a defined spectral range (generally defined by the device supplier). That data is then run through an algorithm to calculate the sample value which is displayed on the device. . . It is important to note that the algorithm calculates the correct value only for clear solutions, with no light scattering from suspended particles (Turbidity). The calculation is based on the Beer’s Lamberts Law which states “The concentration is directly proportional to the absorption of light at a given wave length. Light scattering by particles or bubbles mimics light absorbance, resulting in an incorrect value.
Here is a link to an article written by Kevin Costa of Hanna Instruments that is a good overview of how the Hanna Checkers (Colorimeter) work https://www.reef2reef.com/ams/understanding-colorimetry-and-your-hanna-checker®-hc.341/
ASSESSMENT
Assessment using a digital measuring device is much easier than visual assessment… No color evaluation necessary…Just read the digital output to obtain your value.
SOURCES OF ERROR IN THE TESTING (EVALUATION) PROCEDURE
The sources of error in the testing procedure for digital testing are much the same as the visual testing methods with exception of the Instrument.
1) Inaccurate reagent measurement
a. Inaccurate measuring devices
i. Droppers
ii. Syringes/Pipettes
iii. Spoons
iv. Other
b. Procedural error in reagent measurement
i. Not reading the measurement meniscus (A Parallax Error-See Part 2)
ii. Not holding droppers vertical for dispensing
iii. Air bubbles in drops when dispensing
iv. Measuring Spoon over/under fill
(For Examples See Table 1-6 and Fig 9-10 in PART 2 of this article)
2) Instrument (Meter---Hanna Checkers or other ) Errors
a. Procedural errors with instrument
i.Vials not clean on exterior; finger prints water marks & or scratched
ii. Not positioning the vials the same way every time
iii. Using the same vial for multiple tests. (Label the Vials to prevent this See Fig 21)
iv. Not covering the top of the meter to keep out stray light
v. Inside of vials and caps contaminated and not properly cleaned & rinsed
vi. Micro-Bubbles in test vile causing light scattering
vii. Suspended material in vile causing light scattering
b. Instrument Errors
i. Does not meet the calibration test standard—Out of Calibration
ii. Damaged Instrument
iii. Test Meter not intended for testing salt water
The tables and illustrations below are some examples of how some of the above errors can affect the results. The precision statement is the Mean ± the Range (High Minus Low)
EXTERIOR OF VIAL DIRTY
TABLE 9
EXTERIOR OF VIAL CLEAN
TABLE 9A
RANDOM POSITIONING OF VIAL
TABLE 10
CONSISTENT POSITIONING OF VIAL
TABLE 10A
As you can see from Tables 9 to 10A a clean vial and consistent positioning increases the precision .To help with positioning of the vials I placed a mark on the vial and the Checker. I line them up when I place the vial in the checker. (See Fig 20) I also label the vials to prevent cross contamination. (See Fig 21)

FIG 20


FIG 21
VIAL RINSING & CONTAMINATION
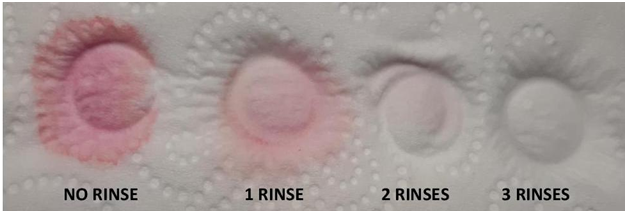
FIG 22
Fig 22 Explanation
Sample vile was emptied then placed on an absorbent material (actually toilet paper) and allowed to drain. It was then rinsed and emptied and again allowed to drain. This was repeated 3 times. At the end of the second rinsing a small amount of contamination is still visible and a third rinse was required. This would indicate a good “best” practice would be 3 rinses. It should also be noted that the cap of the vile needs also to be rinsed because it can be a source of contamination. (Note on contamination: 1ppm is roughly 7 drops in 60 gal so that means a very-- very small amount is required to contaminate a 10 ml test vial--- .000001mL)
CONCLUSIONS ON DIGITAL TESTING METHODS
Many of the same conclusions that we saw in the Visual Testing can be applied to Instrumental (Digital) Testing. The key elements are the same. These are covered in the section, “Establish Good Laboratory Practices (EVALUATION)” in the Visual Testing section Good laboratory “best practices” apply here also. (See PART 3--CONCLUSION ON VISUAL TESTING METHODS) The main difference is the instrument itself.
KNOW YOUR INSTRUMENT:
It is important to understand the performance statistics of the instrument (meter) you are using as well as the instrument care, calibration and correct usage. Here are some things to consider when using an instrument that can help in utilizing “best practices”:
1) What is the stated accuracy?
This important to know the significance of the value you get as a result of a test. Knowing the significance of the value helps to make decisions on any actions that might be necessary. For example the Hanna Calcium Checker has a reported accuracy of ±6% of the reading. If the reading is 400ppm this could actually be 424ppm or 376ppm. This would mean that the “actual” value could be between these two values. One way of better quantifying this value for an individual instrument is to run tests on known solutions or standards. By doing this the accuracy can be better defined. Once this value is know as long as the instrument has good repeatability a correction factor can be applied to get more accurate results. For example, my Calcium Checker consistently measurers’ 15-20 ppm lower when compared to a known standards of 350 & 400 ppm…(4-5%) The key word here is “consistently”. When measuring a sample I simply multiply the value obtained by 1.05 and get my results. Using this method I am consistently within ± 10ppm of external testing sources results.
All of the Hanna Checkers have reported accuracy values. They are stated either a % of the meter reading or as a ± ppm or ppb value plus a % of the meter reading. For example the HI 736 Phosphorous Ultra Low Range has a reported accuracy of ±5ppb ±5% of the reading. A meter reading of 10 would then have a range of 10ppb ±5ppb plus 5% of 10ppb = .5ppb…Meaning the “actual” value would be between 4.5ppb and 15.5ppb. Again by “calibrating” the meter with known solutions the “true” value can better be determined for that particular meter.
It is important to note, accuracy must be determined by measuring against a known sample. Obtaining known standards is a challenge. Reference Calibration Standards for our hobby (seawater) are not easy to come by…Especially certified ones…and they are expensive!...Here is an example https://highpuritystandards.com/elements-in-seawater/ (This is the one used in the article by Rich Ross & Dr. Chris Maupin in Skeptical Reefkeeping). There are other ones that you can get, they are not certified but verified via ICP testing…Like this one https://www.bulkreefsupply.com/fauna-marin-multi-reference-solution.html I generally prepare my own standards. The point is you have to have an good target in order to know if your test is giving accurate results. So the precision can be high (good repeatability and reproducibility) but it is precisely incorrect.
2) What is the precision of the instrument?
Precision is how close two or more measurements are to each other regardless of the accuracy of the measurement. It is important that an instrument be able to measure the same sample multiple times and get the same (or very close) results. If this is not the case it becomes very difficult to establish accuracy.
One method to determine the Precision of an instrument is by measuring the same sample with the instrument multiple times. The values are then added together and then divided by the total number of samples. This gives the Mean (Avg.) of the sample set. The lowest value in the set is subtracted from the highest value in the set to get the “range” The precision is then given as Mean ± Range value. (See Example &Table 11)
Example: HI 736 Phosphorous Ultra Low Range Precision Test
TABLE 11
RANGE = 105-99= 6 PRECISION STATEMENT 101.5 ± 6ppb
The test was conducted using the Hanna Calibration Check Sample Set as the sample to be measured. These sets are available for all of the Hanna Checkers and are useful for precision testing as well as checking to see if the instrument is within its calibration range.
There are other methods to evaluate the precision and if you are interested here is a link to see how they are done. https://www.wikihow.com/Calculate-Precision
3) Is the instrument subject to EMC Deviation?
EMC Deviation is the deviation in accuracy due to Electro-magnetic interference. Using the instrument near an Electro-magnet source (unshielded electric motor, computer screen, some fluorescent light fixtures etc.) can cause errors in the readings. The specifications sheet for the instrument will let you know if this is an issue. For example the HI-736 Phosphorous Checker has an EMC Deviation of ±5ppb.
4) Run periodic calibration checks on the instruments.
Checking to see if the instrument is measuring in an acceptable range will help to insure the dependability of the results. The Hanna Calibration Check Samples are designed for this purpose. I check mine monthly or if I suspect there might be an issue with erratic measurements. Keep a control chart (record) of your calibration results over time. This will help you to track the instruments performance over time and help to spot any trends.
5) Read the operating manual of the Instrument.
Carefully read the operating manual of the instrument. Take note of things like operating environment, calibration requirements, error codes and other operational instructions.
All of these and most likely more are steps to take to insure the measurement is as Accurate & Precise as possible. Having measurements be as Accurate and Precise as possible coupled with best laboratory practices provides reliable information about the water quality of an aquarium. This will give a higher degree of confidence in taking any corrective action required.
BRS did a video on along these lines (Link Below) that also illustrates this point. The one issue I have with their approach is that they defined “Accuracy” as the average of the reading subtracting out the highest and lowest 10% of reading to get the target value. I do not think this defines accuracy. A valid reference standard would have been a better approach, but none the less it is valuable to illustrate the point. https://www.bulkreefsupply.com/video/view/can-you-trust-your-saltwater-test-kit-or-your-testing-skills-on-your-reef-tank-brstv-investigates/?browse_eid=b3e52ff6-9fa6-460d-900f-e636559973d2&utm_source=bronto&utm_medium=email&utm_term=WATCH+VIDEO&utm_content=[VIDEO]+Can+you+trust+your+test+kit?&utm_campaign=Video+071919&_bta_tid=00891137605476391548835156685805154972018283493119219376896555356694754057504034082108396405036106573321
ICP TESTING
ICP testing of salt water has become very popular in the last few years. That being the case a few things to think about with regards to the results obtained from this method. ICP (Inductively Coupled Plasma) Spectroscopy is an analytical method used to detect and measure elements to analyze chemical samples. The process is based on the ionization of a sample by extremely hot plasma, usually made from argon gas. ICP comes in different “flavors”.
ICP AES
Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) or ICP Atomic Emission Spectroscopy is a technique that can determine concentrations of trace to major elements and can detect most elements in the periodic table. Reliable results can be obtained for about 70 elements with detection limits in the parts per billion ranges.
ICP MS
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) or ICP Mass Spectrometry is highly sensitive and capable of multi-element trace analysis and ultra trace analysis, often at the parts-per-trillion level. Testing for trace elements can be performed on a range of materials from super alloys to high purity materials.
ICP OES (This is the method most widely used for testing salt water samples)
ICP-OES (Inductively coupled plasma - optical emission spectrometry) is a technique in which the composition of elements in (mostly water-dissolved) samples can be determined using plasma and a spectrometer.
The important factor to consider with ICP testing is that it is a Method. That means just like the Methods covered above (Visual Testing & Instrumental Testing) it is subject to many of the same errors…Errors in sample preparation…Errors in instrument calibration…Procedural Errors..ETC. Doing an ICP test is not a trivial matter. It is not just a complex instrument that you put your sample in and out pops the answer. It consists of procedures and methods that need to be followed precisely in order to get accurate results. I say this to point out that although ICP tests produce large amounts of numerical data, reporting in ppm and ppb it is subject to errors and incorrect methods just like our Hobby Grade Kits. This mean
that ICP testing is subject to precision and accuracy ranges the same as the previous test discussed. Rich Ross and Dr. Chris Maupin published a great article in Reefs Magazine titled “Skeptical Reefkeeping 12: Triton Lab ICP-OES Testing of a Certified Artificial Saltwater Standard”. This article illustrates this point. ---Here is a link to the article
http://packedhead.net/2015/triton-lab-icp-oes-testing-of-a-certified-artificial-saltwater-standard/
The key points to remember is the ICP testing are subject to variations and errors and therefore are not “absolute” values.
For the last several months I have been sending in my water samples to 3 different ICP test facilities and recording the data for comparison. All 3 facilities state that they have high “accurate” and “precision”. As the article by Ross & Maupin illustrates this is not totally the case in all the areas of analysis. By looking at 3 different ICP services (Identified as ICP 1-3) it becomes apparent that not all ICP testing is created equal…at least from my point of view. The table (Table 12) below is a comparison of 3 different ICP testing facilities testing the same sample. 3 samples were taken on each of the indicated dates and shipped to the ICP test facility on the same day. Many of the values are close, but some are not. The question then becomes which one is correct. This becomes a difficult question to answer because the data does not include any specific accuracy statements for each of the elements tested. From a Quality System perspective this presents a problem. If the reported value is let’s say .1 ppm what is the possible error in this value based on the accuracy statement is it ± .01 ppm? or ± .05 ppm? These two could have completely different responses to the reported value if let’s say we are dealing with Iodine. The Yellow cells in Table 12 are the elements most commonly monitored and dosed for adjustments.
I want to be clear. I am not negative on ICP testing. I think it brings a new level of testing to our hobby. That being said I think a clear statement to as to the expected accuracy for each of the elements would be very helpful in making decisions as to any actions we should be taking based on the results.
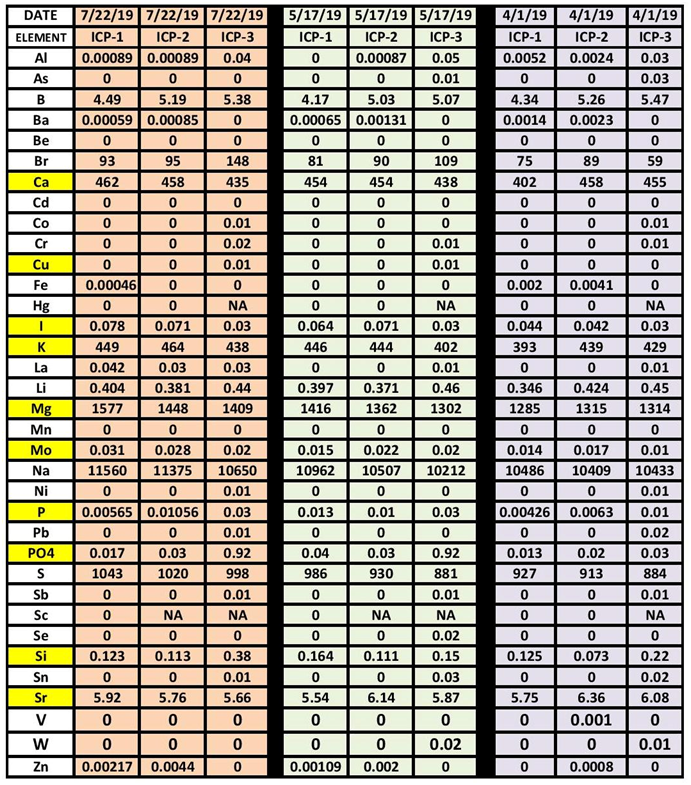
TABLE 12
FINAL CONCLUSIONS
We have covered a great deal in this 4 part series and there is a lot more that could be said. Hopefully you have found some valuable tips to help improve the Accuracy & Precision of your water testing.
As was mentioned in the introduction; Aquarium water testing is an analytical method. Just like any other analytical method it shares the challenge of getting good Accuracy and Precision. By approaching our testing from a Quality Systems mind set, we can improve our accuracy and precision and have confidence in our results to provide our tank critters with the best environment possible.
As we turn our attention to the use of digital testers (Colorimeters) such as the HANNA CHECKER, most of the same elements necessary for good precision and accuracy that exist in Visual Testing also exist in Digital Testing. Digital Testing methods have the same two steps as the Visual method: Evaluation (The Chemistry) and Assessment. Evaluation is the test procedure itself and Assessment is the determination of the results.
TESTING PROCEDURES (EVALUATION)
Most test kits consist of a series of reagents added to the sample according to a prescribed procedure. The procedure generally requires that the reagents be measured out by “drops”, ml or some type of measuring spoon. They are added to the sample being tested in prescribed order. There is usually a time element associated with the procedure…Mix for 1 min…wait for 30 seconds…etc. Part of the procedure using digital tests most often requires the measurement of a “Blank”. The Blank is the initial measurement that is taken in order to compensate for the “color” of the test sample itself (Water Color). After the last step in the procedure the sample is placed into the device for measurement. The device measures the light absorbed by the sample across a defined spectral range (generally defined by the device supplier). That data is then run through an algorithm to calculate the sample value which is displayed on the device. . . It is important to note that the algorithm calculates the correct value only for clear solutions, with no light scattering from suspended particles (Turbidity). The calculation is based on the Beer’s Lamberts Law which states “The concentration is directly proportional to the absorption of light at a given wave length. Light scattering by particles or bubbles mimics light absorbance, resulting in an incorrect value.
Here is a link to an article written by Kevin Costa of Hanna Instruments that is a good overview of how the Hanna Checkers (Colorimeter) work https://www.reef2reef.com/ams/understanding-colorimetry-and-your-hanna-checker®-hc.341/
ASSESSMENT
Assessment using a digital measuring device is much easier than visual assessment… No color evaluation necessary…Just read the digital output to obtain your value.
SOURCES OF ERROR IN THE TESTING (EVALUATION) PROCEDURE
The sources of error in the testing procedure for digital testing are much the same as the visual testing methods with exception of the Instrument.
1) Inaccurate reagent measurement
a. Inaccurate measuring devices
i. Droppers
ii. Syringes/Pipettes
iii. Spoons
iv. Other
b. Procedural error in reagent measurement
i. Not reading the measurement meniscus (A Parallax Error-See Part 2)
ii. Not holding droppers vertical for dispensing
iii. Air bubbles in drops when dispensing
iv. Measuring Spoon over/under fill
(For Examples See Table 1-6 and Fig 9-10 in PART 2 of this article)
2) Instrument (Meter---Hanna Checkers or other ) Errors
a. Procedural errors with instrument
i.Vials not clean on exterior; finger prints water marks & or scratched
ii. Not positioning the vials the same way every time
iii. Using the same vial for multiple tests. (Label the Vials to prevent this See Fig 21)
iv. Not covering the top of the meter to keep out stray light
v. Inside of vials and caps contaminated and not properly cleaned & rinsed
vi. Micro-Bubbles in test vile causing light scattering
vii. Suspended material in vile causing light scattering
b. Instrument Errors
i. Does not meet the calibration test standard—Out of Calibration
ii. Damaged Instrument
iii. Test Meter not intended for testing salt water
The tables and illustrations below are some examples of how some of the above errors can affect the results. The precision statement is the Mean ± the Range (High Minus Low)
EXTERIOR OF VIAL DIRTY
| TEST # | 1 | 2 | 3 | 4 | 5 | PRECISION |
| ppb | 9 | 15 | 21 | 7 | 8 | 12 ± 14 |
EXTERIOR OF VIAL CLEAN
| TEST # | 1 | 2 | 3 | 4 | 5 | PRECISION |
| ppb | 10 | 12 | 9 | 10 | 12 | 10.6 ± 3 |
RANDOM POSITIONING OF VIAL
| TEST # | 1 | 2 | 3 | 4 | 5 | PRECISION |
| ppb | 9 | 13 | 12 | 8 | 10 | 10.4 ± 5 |
CONSISTENT POSITIONING OF VIAL
| TEST # | 1 | 2 | 3 | 4 | 5 | PRECISION |
| ppb | 9 | 10 | 8 | 12 | 9 | 10.2 ± 2 |
As you can see from Tables 9 to 10A a clean vial and consistent positioning increases the precision .To help with positioning of the vials I placed a mark on the vial and the Checker. I line them up when I place the vial in the checker. (See Fig 20) I also label the vials to prevent cross contamination. (See Fig 21)
FIG 20
FIG 21
VIAL RINSING & CONTAMINATION
FIG 22
Fig 22 Explanation
Sample vile was emptied then placed on an absorbent material (actually toilet paper) and allowed to drain. It was then rinsed and emptied and again allowed to drain. This was repeated 3 times. At the end of the second rinsing a small amount of contamination is still visible and a third rinse was required. This would indicate a good “best” practice would be 3 rinses. It should also be noted that the cap of the vile needs also to be rinsed because it can be a source of contamination. (Note on contamination: 1ppm is roughly 7 drops in 60 gal so that means a very-- very small amount is required to contaminate a 10 ml test vial--- .000001mL)
CONCLUSIONS ON DIGITAL TESTING METHODS
Many of the same conclusions that we saw in the Visual Testing can be applied to Instrumental (Digital) Testing. The key elements are the same. These are covered in the section, “Establish Good Laboratory Practices (EVALUATION)” in the Visual Testing section Good laboratory “best practices” apply here also. (See PART 3--CONCLUSION ON VISUAL TESTING METHODS) The main difference is the instrument itself.
KNOW YOUR INSTRUMENT:
It is important to understand the performance statistics of the instrument (meter) you are using as well as the instrument care, calibration and correct usage. Here are some things to consider when using an instrument that can help in utilizing “best practices”:
1) What is the stated accuracy?
This important to know the significance of the value you get as a result of a test. Knowing the significance of the value helps to make decisions on any actions that might be necessary. For example the Hanna Calcium Checker has a reported accuracy of ±6% of the reading. If the reading is 400ppm this could actually be 424ppm or 376ppm. This would mean that the “actual” value could be between these two values. One way of better quantifying this value for an individual instrument is to run tests on known solutions or standards. By doing this the accuracy can be better defined. Once this value is know as long as the instrument has good repeatability a correction factor can be applied to get more accurate results. For example, my Calcium Checker consistently measurers’ 15-20 ppm lower when compared to a known standards of 350 & 400 ppm…(4-5%) The key word here is “consistently”. When measuring a sample I simply multiply the value obtained by 1.05 and get my results. Using this method I am consistently within ± 10ppm of external testing sources results.
All of the Hanna Checkers have reported accuracy values. They are stated either a % of the meter reading or as a ± ppm or ppb value plus a % of the meter reading. For example the HI 736 Phosphorous Ultra Low Range has a reported accuracy of ±5ppb ±5% of the reading. A meter reading of 10 would then have a range of 10ppb ±5ppb plus 5% of 10ppb = .5ppb…Meaning the “actual” value would be between 4.5ppb and 15.5ppb. Again by “calibrating” the meter with known solutions the “true” value can better be determined for that particular meter.
It is important to note, accuracy must be determined by measuring against a known sample. Obtaining known standards is a challenge. Reference Calibration Standards for our hobby (seawater) are not easy to come by…Especially certified ones…and they are expensive!...Here is an example https://highpuritystandards.com/elements-in-seawater/ (This is the one used in the article by Rich Ross & Dr. Chris Maupin in Skeptical Reefkeeping). There are other ones that you can get, they are not certified but verified via ICP testing…Like this one https://www.bulkreefsupply.com/fauna-marin-multi-reference-solution.html I generally prepare my own standards. The point is you have to have an good target in order to know if your test is giving accurate results. So the precision can be high (good repeatability and reproducibility) but it is precisely incorrect.
2) What is the precision of the instrument?
Precision is how close two or more measurements are to each other regardless of the accuracy of the measurement. It is important that an instrument be able to measure the same sample multiple times and get the same (or very close) results. If this is not the case it becomes very difficult to establish accuracy.
One method to determine the Precision of an instrument is by measuring the same sample with the instrument multiple times. The values are then added together and then divided by the total number of samples. This gives the Mean (Avg.) of the sample set. The lowest value in the set is subtracted from the highest value in the set to get the “range” The precision is then given as Mean ± Range value. (See Example &Table 11)
Example: HI 736 Phosphorous Ultra Low Range Precision Test
| MEASUREMENT | 1 | 2 | 3 | 4 | 5 | 6 | MEAN |
| VALUE ppb | 105 | 102 | 99 | 101 | 100 | 102 | 101.5 |
RANGE = 105-99= 6 PRECISION STATEMENT 101.5 ± 6ppb
The test was conducted using the Hanna Calibration Check Sample Set as the sample to be measured. These sets are available for all of the Hanna Checkers and are useful for precision testing as well as checking to see if the instrument is within its calibration range.
There are other methods to evaluate the precision and if you are interested here is a link to see how they are done. https://www.wikihow.com/Calculate-Precision
3) Is the instrument subject to EMC Deviation?
EMC Deviation is the deviation in accuracy due to Electro-magnetic interference. Using the instrument near an Electro-magnet source (unshielded electric motor, computer screen, some fluorescent light fixtures etc.) can cause errors in the readings. The specifications sheet for the instrument will let you know if this is an issue. For example the HI-736 Phosphorous Checker has an EMC Deviation of ±5ppb.
4) Run periodic calibration checks on the instruments.
Checking to see if the instrument is measuring in an acceptable range will help to insure the dependability of the results. The Hanna Calibration Check Samples are designed for this purpose. I check mine monthly or if I suspect there might be an issue with erratic measurements. Keep a control chart (record) of your calibration results over time. This will help you to track the instruments performance over time and help to spot any trends.
5) Read the operating manual of the Instrument.
Carefully read the operating manual of the instrument. Take note of things like operating environment, calibration requirements, error codes and other operational instructions.
All of these and most likely more are steps to take to insure the measurement is as Accurate & Precise as possible. Having measurements be as Accurate and Precise as possible coupled with best laboratory practices provides reliable information about the water quality of an aquarium. This will give a higher degree of confidence in taking any corrective action required.
BRS did a video on along these lines (Link Below) that also illustrates this point. The one issue I have with their approach is that they defined “Accuracy” as the average of the reading subtracting out the highest and lowest 10% of reading to get the target value. I do not think this defines accuracy. A valid reference standard would have been a better approach, but none the less it is valuable to illustrate the point. https://www.bulkreefsupply.com/video/view/can-you-trust-your-saltwater-test-kit-or-your-testing-skills-on-your-reef-tank-brstv-investigates/?browse_eid=b3e52ff6-9fa6-460d-900f-e636559973d2&utm_source=bronto&utm_medium=email&utm_term=WATCH+VIDEO&utm_content=[VIDEO]+Can+you+trust+your+test+kit?&utm_campaign=Video+071919&_bta_tid=00891137605476391548835156685805154972018283493119219376896555356694754057504034082108396405036106573321
ICP TESTING
ICP testing of salt water has become very popular in the last few years. That being the case a few things to think about with regards to the results obtained from this method. ICP (Inductively Coupled Plasma) Spectroscopy is an analytical method used to detect and measure elements to analyze chemical samples. The process is based on the ionization of a sample by extremely hot plasma, usually made from argon gas. ICP comes in different “flavors”.
ICP AES
Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) or ICP Atomic Emission Spectroscopy is a technique that can determine concentrations of trace to major elements and can detect most elements in the periodic table. Reliable results can be obtained for about 70 elements with detection limits in the parts per billion ranges.
ICP MS
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) or ICP Mass Spectrometry is highly sensitive and capable of multi-element trace analysis and ultra trace analysis, often at the parts-per-trillion level. Testing for trace elements can be performed on a range of materials from super alloys to high purity materials.
ICP OES (This is the method most widely used for testing salt water samples)
ICP-OES (Inductively coupled plasma - optical emission spectrometry) is a technique in which the composition of elements in (mostly water-dissolved) samples can be determined using plasma and a spectrometer.
The important factor to consider with ICP testing is that it is a Method. That means just like the Methods covered above (Visual Testing & Instrumental Testing) it is subject to many of the same errors…Errors in sample preparation…Errors in instrument calibration…Procedural Errors..ETC. Doing an ICP test is not a trivial matter. It is not just a complex instrument that you put your sample in and out pops the answer. It consists of procedures and methods that need to be followed precisely in order to get accurate results. I say this to point out that although ICP tests produce large amounts of numerical data, reporting in ppm and ppb it is subject to errors and incorrect methods just like our Hobby Grade Kits. This mean
that ICP testing is subject to precision and accuracy ranges the same as the previous test discussed. Rich Ross and Dr. Chris Maupin published a great article in Reefs Magazine titled “Skeptical Reefkeeping 12: Triton Lab ICP-OES Testing of a Certified Artificial Saltwater Standard”. This article illustrates this point. ---Here is a link to the article
http://packedhead.net/2015/triton-lab-icp-oes-testing-of-a-certified-artificial-saltwater-standard/
The key points to remember is the ICP testing are subject to variations and errors and therefore are not “absolute” values.
For the last several months I have been sending in my water samples to 3 different ICP test facilities and recording the data for comparison. All 3 facilities state that they have high “accurate” and “precision”. As the article by Ross & Maupin illustrates this is not totally the case in all the areas of analysis. By looking at 3 different ICP services (Identified as ICP 1-3) it becomes apparent that not all ICP testing is created equal…at least from my point of view. The table (Table 12) below is a comparison of 3 different ICP testing facilities testing the same sample. 3 samples were taken on each of the indicated dates and shipped to the ICP test facility on the same day. Many of the values are close, but some are not. The question then becomes which one is correct. This becomes a difficult question to answer because the data does not include any specific accuracy statements for each of the elements tested. From a Quality System perspective this presents a problem. If the reported value is let’s say .1 ppm what is the possible error in this value based on the accuracy statement is it ± .01 ppm? or ± .05 ppm? These two could have completely different responses to the reported value if let’s say we are dealing with Iodine. The Yellow cells in Table 12 are the elements most commonly monitored and dosed for adjustments.
I want to be clear. I am not negative on ICP testing. I think it brings a new level of testing to our hobby. That being said I think a clear statement to as to the expected accuracy for each of the elements would be very helpful in making decisions as to any actions we should be taking based on the results.
TABLE 12
FINAL CONCLUSIONS
We have covered a great deal in this 4 part series and there is a lot more that could be said. Hopefully you have found some valuable tips to help improve the Accuracy & Precision of your water testing.
As was mentioned in the introduction; Aquarium water testing is an analytical method. Just like any other analytical method it shares the challenge of getting good Accuracy and Precision. By approaching our testing from a Quality Systems mind set, we can improve our accuracy and precision and have confidence in our results to provide our tank critters with the best environment possible.
Last edited by a moderator:












