I've got a question that I cant seem to find a good answer to, and I know someone on here will have one! ;-)
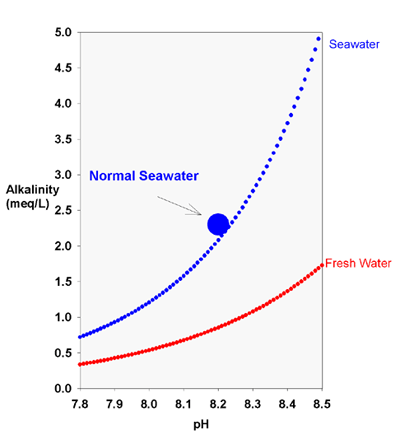
Lets say we have a reef tank, running a high ph salt, for example red sea coral pro that at 35ppt mixes to 8.5 pH. Then say we took that reef tank and stuck it outside in the country and left it there to equalize its o2/co2 with the outside air. What is the practical maximum pH we could see? What about theoretical?
edit: changed would to could
Lets say we have a reef tank, running a high ph salt, for example red sea coral pro that at 35ppt mixes to 8.5 pH. Then say we took that reef tank and stuck it outside in the country and left it there to equalize its o2/co2 with the outside air. What is the practical maximum pH we could see? What about theoretical?
edit: changed would to could
Last edited: