- Joined
- Apr 25, 2016
- Messages
- 759
- Reaction score
- 438
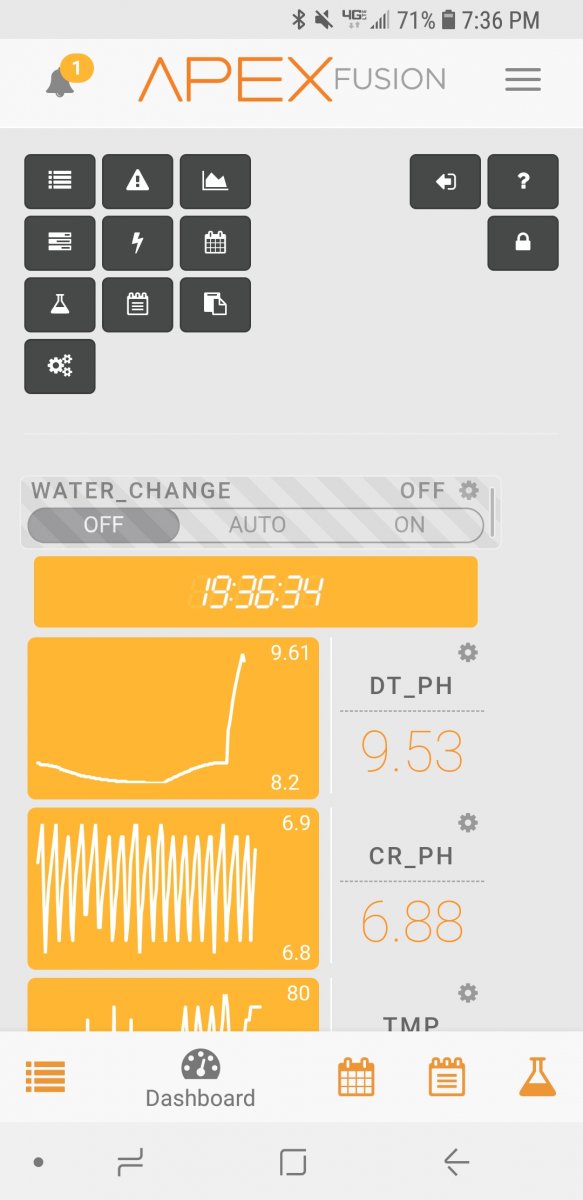
Setup a recirculating c02 scrubber on my system a few weeks ago. Asked my wife to feed the tank some food as I didnt have time to do it before I left for work. I guess the skimmer started overflowing and now I have a closed loop running water from the collection cup, through the soda lime media and back into the skimmer input. Ph shot up and I can assume my kh did the same thing. Wont be home for a few hours, don't think I've stressed this much in a long long time...