GoVols
Cobb / Webb - 1989
View Badges
Reef Squad Leader
Excellence Award
Reef Tank 365
MTRCMember
Article Contributor
Hospitality Award
R2R Research
My Tank Thread
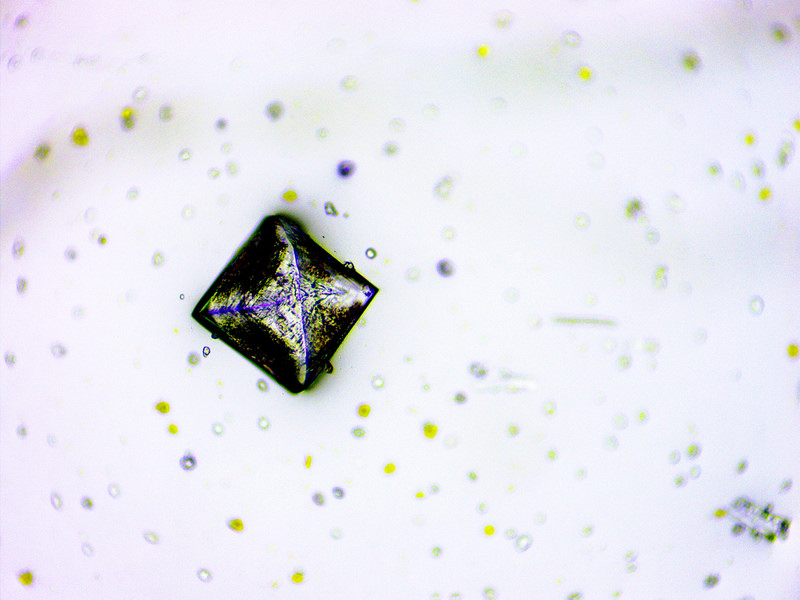
When a single crystal is formed.
Does it contain the big-3 and trace elements or is there different kind of crystals or particles with in a salt mix?
Thanks, GoVols
Does it contain the big-3 and trace elements or is there different kind of crystals or particles with in a salt mix?
Thanks, GoVols