- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
This is about Seachem Clarity and its use for water clarifying. I don't know exactly what it is, but I know a common guess that it is not, and I thought I'd post a little about how it seems to behave.
The manufacturer page describes it as "...the ultimate clarifier for both fresh and saltwater. It employs an advanced polymeric flocculating agent that is both reef and plant safe. ... Clarity™ will clear all types of clouding."
The SDS sheet (attached) says
"* Proprietary aqueous solution of iron salt and other ingredients, including polymeric flocculating agent, which are non - hazardous or less than 1% in concentration (or 0.1% for carcinogens, reproductive toxins, or respiratory sensitizers). Specific chemical identity of salt, polymers and other ingredients and exact percentage concentration withheld as proprietary trade secrets."
First, there is a widely used polymer flocculant in drinking water - polyDADMAC (wiki). It has been suggested in a few places (including by me... oops) that maybe aquarium clarifiers like Clarity use this polymer. I don't know about others, but Clarity looks like it's NOT a cationic polymer.
PolyDADMAC is a polyquat or cationic polymer with a high charge density. I have used several different methods that detect or quantify polyquats / cationic polymers. Clarity does not behave like it contains any cationic polymer at all in response to any of these methods: SDS, bromophenol blue, or toluidine blue o titration. The last method is used in fairly standard test kits (Lamotte) for polyquat measurement, and has been used on polyDADMAC specifically.
Here's what I did find out about how Clarity behaves. It is very acidic in the bottle (below pH of 3) , and as soon as you bring the pH up even slightly (above 4), it forms solids and falls out of solution. This would happen in fresh or saltwater aquaria.
Here's what happens with a dilute solution of Clarity (3%) in distilled water as you bring the pH up with dropwise addition of 1M NaOH...
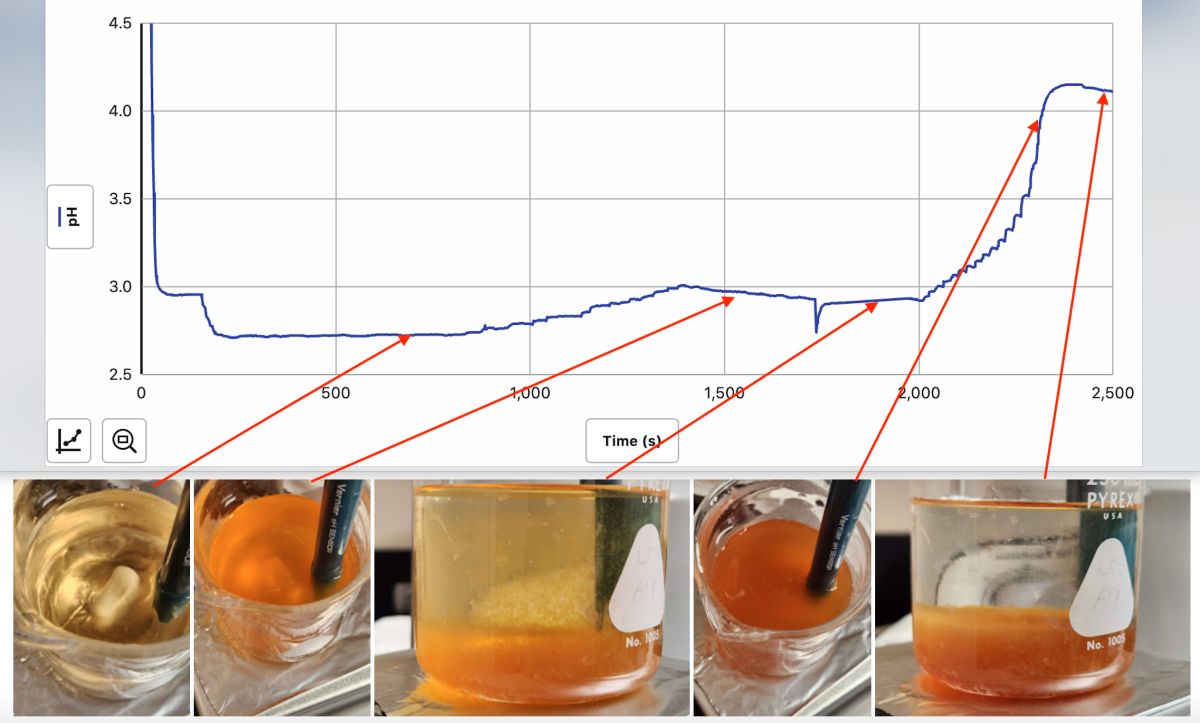
adding the clarity drops the pH below 3. Stirring alone for 10 minutes does nothing - the solution remains light brown (1st pic from left). Adding NaOH drops does little to raise the pH initially, but creates darker colored solids that settle out of the still yellow liquid when the stirring is turned off (pics 2 and 3). Eventually the pH starts to rise quickly with each drop and more NaOH no longer precipitates solids, and the remaining water after settling is totally clear (pics 4 and 5).
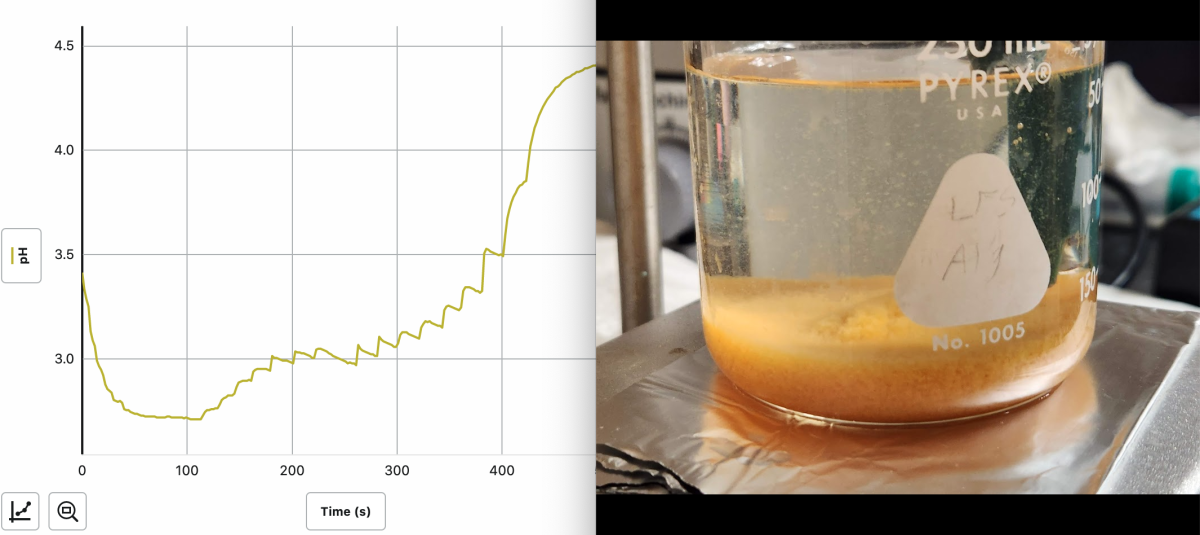
This worked the same when adding saturated sodium bicarbonate solution instead of NaOH, see below...
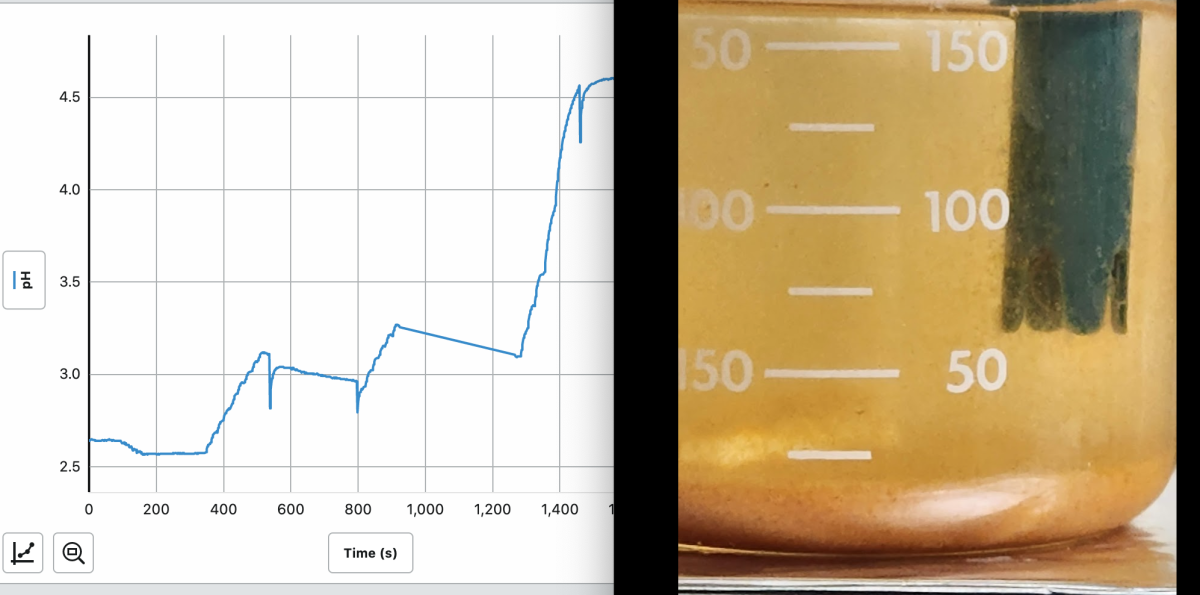
And it worked the same in tank water. I took aquarium water, lowered the pH to ~2.5 with HCl before adding Clarity.
It was totally stable without precipitate until the pH was raised back up with NaOH. The precipitation occurred and was finished before the pH got even to 5.
Since the pH will obviously go above ~4 with any addition to fresh or saltwater aquaria, it seems this mechanism will always be triggered.
This also means that the binding mechanism may not involve being cationic (+) and thus would not be expected to bind to negative surfaces in aquaria. The solids seem loose, fluffy and seachem says they need to be filtered.
"Q: I added the correct amount of Clarity™ to my tank and the cloudiness got much worse. What is happening?
A: Clarity™ is a flocculant, meaning it bonds to small particles, making them much larger and easier to filter from the water. This will make the cloudiness worse for a short time until the large particles are removed from the water. Mechanical filtration is the only way to remove them, and if you don't have adequate mechanical filtration it will probably never clear...."
If your water clarifier product is extremely low pH and precipitates well before the pH reaches aquarium levels, then it might be using a similar mechanism / chemical.
(edit: I'm not a polymer chemist or chemist of any sort, so people with actual expertise may have a different interpretation.)
The manufacturer page describes it as "...the ultimate clarifier for both fresh and saltwater. It employs an advanced polymeric flocculating agent that is both reef and plant safe. ... Clarity™ will clear all types of clouding."
The SDS sheet (attached) says
"* Proprietary aqueous solution of iron salt and other ingredients, including polymeric flocculating agent, which are non - hazardous or less than 1% in concentration (or 0.1% for carcinogens, reproductive toxins, or respiratory sensitizers). Specific chemical identity of salt, polymers and other ingredients and exact percentage concentration withheld as proprietary trade secrets."
First, there is a widely used polymer flocculant in drinking water - polyDADMAC (wiki). It has been suggested in a few places (including by me... oops) that maybe aquarium clarifiers like Clarity use this polymer. I don't know about others, but Clarity looks like it's NOT a cationic polymer.
PolyDADMAC is a polyquat or cationic polymer with a high charge density. I have used several different methods that detect or quantify polyquats / cationic polymers. Clarity does not behave like it contains any cationic polymer at all in response to any of these methods: SDS, bromophenol blue, or toluidine blue o titration. The last method is used in fairly standard test kits (Lamotte) for polyquat measurement, and has been used on polyDADMAC specifically.
Here's what I did find out about how Clarity behaves. It is very acidic in the bottle (below pH of 3) , and as soon as you bring the pH up even slightly (above 4), it forms solids and falls out of solution. This would happen in fresh or saltwater aquaria.
Here's what happens with a dilute solution of Clarity (3%) in distilled water as you bring the pH up with dropwise addition of 1M NaOH...
adding the clarity drops the pH below 3. Stirring alone for 10 minutes does nothing - the solution remains light brown (1st pic from left). Adding NaOH drops does little to raise the pH initially, but creates darker colored solids that settle out of the still yellow liquid when the stirring is turned off (pics 2 and 3). Eventually the pH starts to rise quickly with each drop and more NaOH no longer precipitates solids, and the remaining water after settling is totally clear (pics 4 and 5).
This worked the same when adding saturated sodium bicarbonate solution instead of NaOH, see below...
And it worked the same in tank water. I took aquarium water, lowered the pH to ~2.5 with HCl before adding Clarity.
It was totally stable without precipitate until the pH was raised back up with NaOH. The precipitation occurred and was finished before the pH got even to 5.
Since the pH will obviously go above ~4 with any addition to fresh or saltwater aquaria, it seems this mechanism will always be triggered.
This also means that the binding mechanism may not involve being cationic (+) and thus would not be expected to bind to negative surfaces in aquaria. The solids seem loose, fluffy and seachem says they need to be filtered.
"Q: I added the correct amount of Clarity™ to my tank and the cloudiness got much worse. What is happening?
A: Clarity™ is a flocculant, meaning it bonds to small particles, making them much larger and easier to filter from the water. This will make the cloudiness worse for a short time until the large particles are removed from the water. Mechanical filtration is the only way to remove them, and if you don't have adequate mechanical filtration it will probably never clear...."
If your water clarifier product is extremely low pH and precipitates well before the pH reaches aquarium levels, then it might be using a similar mechanism / chemical.
(edit: I'm not a polymer chemist or chemist of any sort, so people with actual expertise may have a different interpretation.)


















