- Joined
- Feb 10, 2014
- Messages
- 719
- Reaction score
- 578
My turn to throw my data in. Somehow USPS lost my first shipment, but the second was received. I got the results back, and in the interest of fostering community knowledge, here they are.
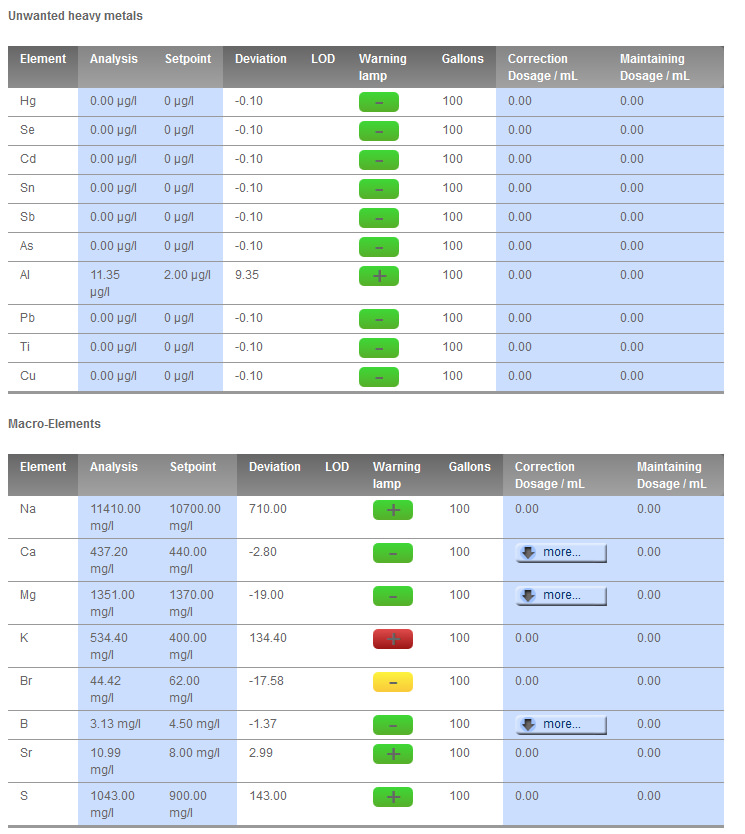
Personal observations: I was running full zeovit previously, which constantly drained my K. I took the reactor offline and went to Ecobak plus biopellets. It appears that I no longer need to dose K! Interesting that AWT and Triton K testing seems to differ quite a bit. AWT always reads K very low for me (-50). I have 3 home test kits, Salifert (winner!), Red Sea (slow), and KZ (useless!). I'm going to stick with Salifert for home K testing. Also, I'm glad to see I am rid of Cu, which I think came in on a rock or something. Had inexplicable STN for a while there. I leave a bag of Cuprisorb in, just in case, these days. I see no downside. Like others stateside, I have mildly raised Li.
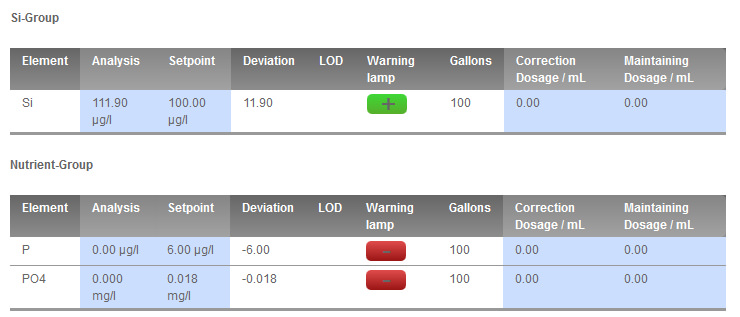
I'm also surprised about showing 0 P, and therefor PO4. I am not, nor have I ever, used any sort of PO4 adsorbent in thise tank. Even AWT always showed my values as low, but 0? Weird. At least my Hanna PO4 egg agrees, right? oh:
oh:
 wangspeed-triton-201412-01
wangspeed-triton-201412-01
 wangspeed-triton-201412-02
wangspeed-triton-201412-02
 wangspeed-triton-201412-03
wangspeed-triton-201412-03
Personal observations: I was running full zeovit previously, which constantly drained my K. I took the reactor offline and went to Ecobak plus biopellets. It appears that I no longer need to dose K! Interesting that AWT and Triton K testing seems to differ quite a bit. AWT always reads K very low for me (-50). I have 3 home test kits, Salifert (winner!), Red Sea (slow), and KZ (useless!). I'm going to stick with Salifert for home K testing. Also, I'm glad to see I am rid of Cu, which I think came in on a rock or something. Had inexplicable STN for a while there. I leave a bag of Cuprisorb in, just in case, these days. I see no downside. Like others stateside, I have mildly raised Li.
I'm also surprised about showing 0 P, and therefor PO4. I am not, nor have I ever, used any sort of PO4 adsorbent in thise tank. Even AWT always showed my values as low, but 0? Weird. At least my Hanna PO4 egg agrees, right?
 wangspeed-triton-201412-01
wangspeed-triton-201412-01 wangspeed-triton-201412-02
wangspeed-triton-201412-02 wangspeed-triton-201412-03
wangspeed-triton-201412-03








