Bruce Carlson published a paper in the late 90's on various tanks at the Waikiki. The 'office' tank used ozone and it was 'clean' (my word, not his. I'll try to find that paper.Other than skimming, UV light and ozone also reduce the yellow-ness of water, but I wonder if the oxidation and UV only modify the structure of the organic molecules that absorb light, OR do they actually change the availability of dissolved organic material for organisms to consume.
In other words, do they make the water cleaner (lower in organic nutrients) or just clearer (less yellow)?
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Whats the best way to determine DOC (dissolved organic compounds)?
- Thread starter chaostactics
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,574
- Reaction score
- 10,155
I wanted to circle back to this question...
I ran across this while thinking about something else, that seems to address it directly.
From Marine Photochemistry and its impact on Carbon cycling
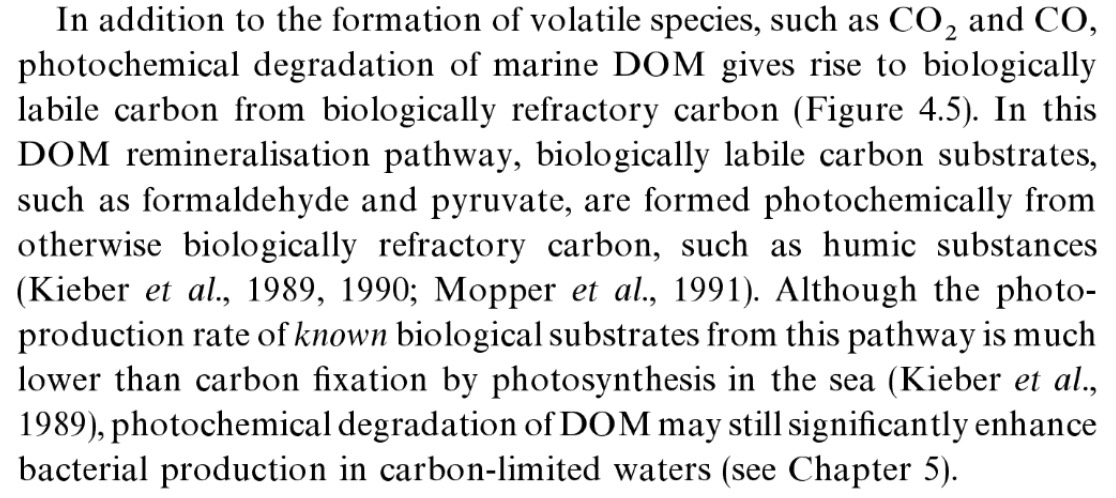
To unpack this a little bit. DOM = dissolved organic matter. Elsewhere it clarifies that matter it's discussing here is specifically the yellowing substances in marine water, and that this can be photo-degraded by light in the UV/Violet/Blue. This photo-degradation happens in part due to reactive species like hydroxyl and other oxidizers that are formed when this light is absorbed by the DOM.
So it's quite possible that UV or oxidizers in our systems are turning yellow refractory (hard to use) oganics into labile (easily consumed) carbon sources.
UV light and ozone also reduce the yellow-ness of water, but I wonder if the oxidation and UV only modify the structure of the organic molecules that absorb light, OR do they actually change the availability of dissolved organic material for organisms to consume.
In other words, do they make the water cleaner (lower in organic nutrients) or just clearer (less yellow)?
Bruce Carlson published a paper in the late 90's on various tanks at the Waikiki. The 'office' tank used ozone and it was 'clean' (my word, not his.
I ran across this while thinking about something else, that seems to address it directly.
From Marine Photochemistry and its impact on Carbon cycling
To unpack this a little bit. DOM = dissolved organic matter. Elsewhere it clarifies that matter it's discussing here is specifically the yellowing substances in marine water, and that this can be photo-degraded by light in the UV/Violet/Blue. This photo-degradation happens in part due to reactive species like hydroxyl and other oxidizers that are formed when this light is absorbed by the DOM.
So it's quite possible that UV or oxidizers in our systems are turning yellow refractory (hard to use) oganics into labile (easily consumed) carbon sources.
- Joined
- Sep 21, 2018
- Messages
- 6,712
- Reaction score
- 7,191
Nice find. I see you are using your free time wiselyI wanted to circle back to this question...
I ran across this while thinking about something else, that seems to address it directly.
From Marine Photochemistry and its impact on Carbon cycling

To unpack this a little bit. DOM = dissolved organic matter. Elsewhere it clarifies that matter it's discussing here is specifically the yellowing substances in marine water, and that this can be photo-degraded by light in the UV/Violet/Blue. This photo-degradation happens in part due to reactive species like hydroxyl and other oxidizers that are formed when this light is absorbed by the DOM.
So it's quite possible that UV or oxidizers in our systems are turning yellow refractory (hard to use) oganics into labile (easily consumed) carbon sources.
Ozone also breaks up big molecules. At one time ozone in a skimmer was a “thing” because it was thought that these smaller molecules were more likely to be removed by the skimmer. I think @Randy Holmes-Farley will have this in his memory banks, might even be in one of his articles.
- Joined
- May 22, 2016
- Messages
- 6,574
- Reaction score
- 10,155
Hah. I'm trying to catch up a bit on my reading list. Since the baby joined us in June, I've been doing night duty. Doing experiments works fine, but any time I would sit down to read something, I fall asleep in 5 minutes.Nice find. I see you are using your free time wisely
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,516
- Reaction score
- 63,963
Ozone certainly can make refractory molecules more bioavailable (and certainly less light absorbing), but it does not necessarily reduce concentrations.
Here's a section from one of my ozone articles:
In a marine mammal pool,18 for example, it was found that disinfection with 4 ppm ozone with a 30 minute contact time (a disinfection level much higher than is typically used in reef aquaria) did not reduce the pool's total organic carbon (TOC) (~13 ppm TOC), while the use of granular activated carbon (GAC) did reduce it by 37%. Interestingly, the combination of ozone and GAC was even more effective, removing 60-78% of the TOC, suggesting that the ozonation may have altered some of the molecules in a way that made them bind more strongly (or more rapidly) to GAC. An alternative explanation that cannot be ruled out involves biological transformations of the organic compounds taking place on the GAC surface as it became colonized with bacteria).
This is the reference cited:
18. Effects of tertiary methods on total organic carbon removal in saline, closed-system marine mammal pools. Adams G; Spotte S. American journal of veterinary research (1980), 41(9), 1470-4.
Here's a section from one of my ozone articles:
In a marine mammal pool,18 for example, it was found that disinfection with 4 ppm ozone with a 30 minute contact time (a disinfection level much higher than is typically used in reef aquaria) did not reduce the pool's total organic carbon (TOC) (~13 ppm TOC), while the use of granular activated carbon (GAC) did reduce it by 37%. Interestingly, the combination of ozone and GAC was even more effective, removing 60-78% of the TOC, suggesting that the ozonation may have altered some of the molecules in a way that made them bind more strongly (or more rapidly) to GAC. An alternative explanation that cannot be ruled out involves biological transformations of the organic compounds taking place on the GAC surface as it became colonized with bacteria).
This is the reference cited:
18. Effects of tertiary methods on total organic carbon removal in saline, closed-system marine mammal pools. Adams G; Spotte S. American journal of veterinary research (1980), 41(9), 1470-4.
Similar threads
- Replies
- 31
- Views
- 927
- Replies
- 12
- Views
- 246
- Replies
- 31
- Views
- 866
- Replies
- 5
- Views
- 148
- Replies
- 2
- Views
- 122












