Can't wait until I figure out what that key to a successful tank is lol.Fair enough, but the reality is, the majority of successful tanks don't have low nutrients. Plenty do, but just as many don't.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How long before vinegar lowers nitrates?
- Thread starter TWYOUNG
- Start date
- Tagged users None
I do 25 gal water changes monthly. I'm experimenting with carbon dosing bc it apparently works for many people and would be far less labor intensive and costly than doing that much water changing. A water change would mess with all my parameters, some of which I don't wish to reduce, phosphate for instance.
I just ventured into larger tanks and completed 125 gallon build, so I understand why you would want to avoid water changes if possible.
A water change is a great way to push the reset button.
Can't wait until I figure out what that key to a successful tank is lol.
Time, stability and knowing who to listen to. no3 is a waste product. So is po4. Having them does not really mean anything. Corals, algae and other things in your tank need nitrogen and phosphorous. While some things can use nitrate for nitrogen, most corals cannot and those that do have to convert nitrate back to ammonia at a large cost of additional energy (like 30-70% but nobody knows for sure). The key is to feed the fish a lot and export a lot. Have available forms of things for the corals and stuff to use, not residual (waste products). Having very low residual no3 and po4 means nothing if there is tons of available forms - you cannot test for the available forms which is why most hobbyists don't even know about them.
Good through put by feeding a lot, exporting a lot. Wide spectrum light from about 350-850nm (more than the visible range). Diverse ecosystem using ocean live rock -bacteria and pods and stuff in a bottle is only a drop in the bucket. Those are the keys, IMO.
I think that the others handled the po4 binding to aragonite. The more that you add, the more that the aragonite binds - this is why I am never an advocate to add a bunch of po4 in the beginning - not only does it bind to the rocks and you have to remove it later, a low po4 level is not an issue if you have other available forms. If you need to lower it, it will lower if you keep using media, but it just takes a while since the rock can bind so much. At low levels, the rock is a buffer... at high levels, it is a reservoir.
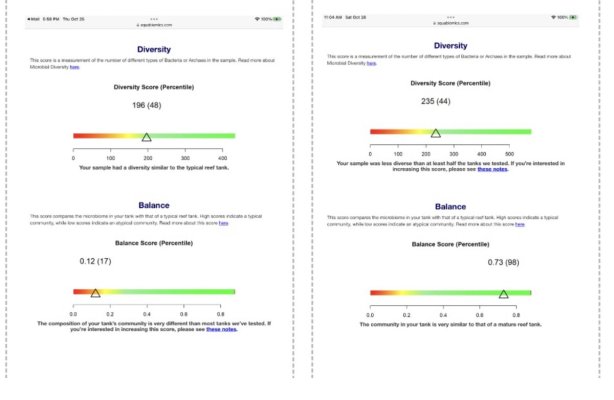
Thanks for the detailed response. I do have two aspects of that I'd like to comment on. Firstly I've been told it's untrue that rock leaches the phosphate you've dosed back into the system. Do you have any documentation for that? Secondly, I made the questionable decision to start my tank 18months ago with dry rock. After encountering dinoflagellates about three months in I began introducing biodiversity through multiple forms of bottled bacteria, AF Lifesource mud, pods, tons of phyto., dirty water, rock and coral rubble from my 30yo FOWLER tank. At this point I could add some live rock but don't feel the benefit outweighs the risks. Attached are biome reports on my tank from about nine months ago, and last month. Although I'm aware these tests have their limits it does seem like a significant improvement.

Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,421
- Reaction score
- 63,782
Im not sure what you heard or exactly what you are questioning, but it is an established and well known, published scientific fact that bare calcium carbonate surfaces reversibly bind phosphate. The higher the concentration in the water, the more binds. When you try to lower the concentration in the water, some of the bound phosphate will come off again to establish a new equilibrium amount of binding.Thanks for the detailed response. I do have two aspects of that I'd like to comment on. Firstly I've been told it's untrue that rock leaches the phosphate you've dosed back into the system. Do you have any documentation for that? Secondly, I made the questionable decision to start my tank 18months ago with dry rock. After encountering dinoflagellates about three months in I began introducing biodiversity through multiple forms of bottled bacteria, AF Lifesource mud, pods, tons of phyto., dirty water, rock and coral rubble from my 30yo FOWLER tank. At this point I could add some live rock but don't feel the benefit outweighs the risks. Attached are biome reports on my tank from about nine months ago, and last month. Although I'm aware these tests have their limits it does seem like a significant improvement.

Last edited:
Probably a term issue. The po4 will not leech from the rock, but it does unbind. This is probably the same to most people, but the difference is pretty big. In the end, if you lower the amount of po4 in the water column, more will leave the rock and enter the water column as it unbinds. Leeching is different - think of leech as uncontrolled whereas binding and unbinding has rules and control and stuff. The po4 bind to aragonite is not permanent.
To me about the only risks of real live rock is a shrimp or crab - easy to catch in a bottle trap... like a walk in the park for somebody who has been dealing with dinos or hair algae. The things that you need are not limited to what shows up in a aquabiomics report... starfish, worms, pods, algae (good), sponges, starfish, etc. are all good. You can get some of these things in mud and other products, but they don't have bottle forms. What is really important is that the rocks already have their real estated occupied by other things so that dinos cannot move into the neighborhood first - the moving trucks and dudes with hand trucks are faster for dinos than the movers for the things that people seem to want.
To me about the only risks of real live rock is a shrimp or crab - easy to catch in a bottle trap... like a walk in the park for somebody who has been dealing with dinos or hair algae. The things that you need are not limited to what shows up in a aquabiomics report... starfish, worms, pods, algae (good), sponges, starfish, etc. are all good. You can get some of these things in mud and other products, but they don't have bottle forms. What is really important is that the rocks already have their real estated occupied by other things so that dinos cannot move into the neighborhood first - the moving trucks and dudes with hand trucks are faster for dinos than the movers for the things that people seem to want.
If po4 binds to a surface and then is covered by live coralline or some coral and no longer has access to the water column either directly or through the porous structure, then it can become trapped. If this rock ends up dying and being dissolved, then the po4 will unbind as it is exposed to the surface or water again. This is likely a rounding error and not a situation for most to be concerned with, but since we are getting all technical and stuff with leeching and unbinding, it felt right...
I use the principle to reduce phos in my tank here;I'd like to see some experimental evidence

Desorbing phosphate from sand with lanthanum.
Finally got around to a hap hazard test for desorbing sand with lanthanum. The sand has been churning around in my fluidised media reactor for a week on a tank currently measuring 0.6 ppm. From previous measurements in the Aragonite adsorption thread, Dan-P kindly calculated my sand had adsorbed...
 www.reef2reef.com
www.reef2reef.com
Accordingly it would seem if I can maintain a steady level where I want it to be for long enough the substrate will reach equilibrium with that level and tend to maintain it. Within reason, should the water level try to go up or down the substrate would release or reabsorb as needed to maintain that level.I use the principle to reduce phos in my tank here;

Desorbing phosphate from sand with lanthanum.
Finally got around to a hap hazard test for desorbing sand with lanthanum. The sand has been churning around in my fluidised media reactor for a week on a tank currently measuring 0.6 ppm. From previous measurements in the Aragonite adsorption thread, Dan-P kindly calculated my sand had adsorbed...www.reef2reef.com
Similar threads
- Replies
- 7
- Views
- 153
- Replies
- 22
- Views
- 882
- Replies
- 12
- Views
- 340
- Replies
- 4
- Views
- 119
- Replies
- 16
- Views
- 293















