Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How to chemically increase nitrates
- Thread starter Pete polyp
- Start date
- Tagged users None
This is actually my barebottom. I have fuge with cheato and tukani live rock. The cheato won't pull out all the phosphate because there's no nitrates...
So if I understand you correctly, you have a high phosphate issue that you are unable to resolve while relying on macro algae to consume it, and that is because your nitrates are too low?
Nitrate is the final step to the nitrogen cycle, it consumes the others from what I understand and have read. I had a tank I ran biopellets on which started with a nitrate of around 20 and 0 phosphate. Before I broke the tank down the nitrate went to 0 and phosphate kept climbing, I think the ratio of pellets is like 16-1 or something like that. A tank with a little nitrate and controlled phosphate actually gives better color and healthier coral imo.
So if I understand you correctly, you have a high phosphate issue that you are unable to resolve while relying on macro algae to consume it, and that is because your nitrates are too low?
The phosphates aren't "high" but elevated slightly. Increased phosphate removal by the cheato would be one desirable side effect of having a little nitrate. I am having to feed so heavily that there's a large amount of phosphate being imported. The main reason I'm trying to increase the nitrate is exactly what JOKER just said, increased color for my sps.
Are you combating PO4 buildup (something else?), or simply trying to maintain some arbitrary nitrate level? Just curious!
-Matt
redfield ratio is not for maintaining an arbitrary level. based on that ratio, both PO4 and NO3 move in sync at that given ratio. I forgot what that exact number is but it's easily searched. Since the lower number is NO3, if you are nitrate limited, your PO4 won't go down anymore. So the theory is to increase NO3 so that the PO4 can drop (together w/ the NO3). Once you bet back to 0, you can add more NO3 and continue to cycle.
What are you using for kno3?
I use Spectracide Stump Remover from Lowes. It will last you a lifetime and super cheap. It's pure KNO3. You can get lab grade online but not necessary.
That's what I was thinking you were using. I'm leaning towards using potassium nitrate because instant ocean needs a little bit of potassium dosing anyways. I'm dosing kalk in this tank, so calcium nitrate would mess my CA/alk balance all up.
redfield ratio is not for maintaining an arbitrary level. based on that ratio, both PO4 and NO3 move in sync at that given ratio. I forgot what that exact number is but it's easily searched. Since the lower number is NO3, if you are nitrate limited, your PO4 won't go down anymore. So the theory is to increase NO3 so that the PO4 can drop (together w/ the NO3). Once you bet back to 0, you can add more NO3 and continue to cycle.[...]
I agree in part, but I don't know that's what the Redfield ratio indicates (nutrient levels "moving with each other"). I do see people using it that way in reefing occasionally.
I think Redfield can be used as a guideline to know when a system is out of balance, but I'm not sure it's an absolute measure even then. I believe you need a baseline to compare with for a given ecosystem to be able to form judgements about "good" or "bad" levels.
It's all theory (based mostly on phytoplankton), really....and useful....I just don't think it offers any specific guidance other than what we already knew:
- We have too much PO4.
- The ocean appears to have an unlimited ability to sequester-at-depth and off-gas phosphate.
- We appear to have water changes.
- ...and a lot of theories that mostly-work, most of the time...at least in theory and when you aren't experiencing one of the many downsides.
Redfield ratio - Wikipedia, the free encyclopedia
...and an interesting quote:
However, looking at the composition of individual species of phytoplankton grown under nitrogen or phosphorus limitation shows that this nitrogen to phosphorus ratio can vary anywhere from 6:1 to 60:1.
and
Although the Redfield ratio is remarkably stable in the deep ocean, phytoplankton may have large variations in the C:Ncomposition, and their life strategy play a role in the C:N
ratio, which has made some researchers speculate that the Redfield ratio perhaps is a general average rather than specific requirement for phytoplankton growth
and a graphic from the article...though the subtext is everything to us (even the ocean has a phosphate issue!):
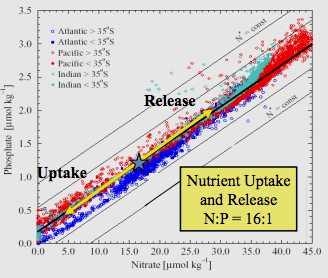
"Relationship of Phosphate to Nitrate Uptake for photosynthesis in various regions of the ocean. Note that nitrate is more often limiting than phosphate"
To take you even further down the rat-hole, consider that some versions of Redfield now includes Iron in the ratio...I can't imagine Zinc will be far behind.
-Matt
That's what I was thinking you were using. I'm leaning towards using potassium nitrate because instant ocean needs a little bit of potassium dosing anyways. I'm dosing kalk in this tank, so calcium nitrate would mess my CA/alk balance all up.
not that much. It takes very little calcium nitrate to maintain 5ppm or so nitrate.
I agree with all the analysis here expecially the 16:1 ratio. What can/does happen is the tank starts having cyano and micro algae issues. One way when that happens is to kill the lights and suspend feeding until the cyano/cloudiness dies off. Then resume with less lights and feeding until a balance happens. IMHO cyano can step up in a low nitrate system but with balanced lighting it dies off at night which returns nitrates back to the system. You basically pump nitrogen gas out of the system and the die off returns nitrates for the corals, coralline, clams, corals and so on. IMHO this is one of the "bad" aspects of DSB operations where at the surface of the substrate you have a low nitrate, low oxygen, high co2 environment.
Finally I have seen some balling (enhanced or balling +) system where a little calcium nitrates is part of the dosing schemes.
my .02
ps besided potassium nitrate I believe is controlled as being possible components of explosives much like ammonia nitrate. (but not sure)
I agree in part, but I don't know that's what the Redfield ratio indicates (nutrient levels "moving with each other"). I do see people using it that way in reefing occasionally.
I think Redfield can be used as a guideline to know when a system is out of balance, but I'm not sure it's an absolute measure even then. I believe you need a baseline to compare with for a given ecosystem to be able to form judgements about "good" or "bad" levels.
I agree w/ you but I think in this case and my own, it's the conclusion (anecdotal) that in order to lower PO4, we need to increase NO3. The baseline fact of high PO4 is already established, the redfield ratio is just used as a guideline to a technique to lower nutrients but not as a way to lower nutrients. Hope I'm making myself clear.
not that much. It takes very little calcium nitrate to maintain 5ppm or so nitrate.
This is true also for KNO3. The K part is negligible. Again, based on my own experience and experience of others, not necessarily science.
I agree w/ you but I think in this case and my own, it's the conclusion (anecdotal) that in order to lower PO4, we need to increase NO3. The baseline fact of high PO4 is already established, the redfield ratio is just used as a guideline to a technique to lower nutrients but not as a way to lower nutrients. Hope I'm making myself clear.[...]
Crystal!
Plankton's role in Redfield's ratio seems like it should be getting more attention, esp. regarding uptake of PO4. Seems like algae and plankton go together in that process. Cultivating plankton maybe should be job #1 in the refugium...along with whatever algae (micro/macro) will grow. (Carbon dosing seems like a very small piece of this puzzle.)
Now back to the topic of dosing nitrates!
(Already gave my $0.02 on how to gauge a first dose before hitting the livestock tank.)
-Matt
Yes Matt, thank you for the idea of mixing some of the solution with water and checking. I'm going to use 1 gallon to check and see exactly how much the solution will change the nitrate
can't remember the exact amount but on my old 55g it must have been only a tablespoon or so of calcium nitrate bumped up nitrate to 5-10ppm. It dropped back down in a week or so in my system which was balanced out with macro algae.
my .02
my .02
I asked this same question a while ago, I ended up using Sodium Nitrate as I didn't want to increase my potassium levels by dosing potassium nitrate. Here is a link to my thread on how to create a solution of Sodium Nitrate.
https://www.reef2reef.com/forums/reef-chemistry-forum/159894-1-mole-sodium-nitrate-solution.html
https://www.reef2reef.com/forums/reef-chemistry-forum/159894-1-mole-sodium-nitrate-solution.html
Stump remover, use the following calculator. Aquatic Plants Fertilization Calculator | Aquarium Tools
The most common I have read about is sodium nitrate. If I had to guess without looking its because sodium is probably one of the more abundant elements in salt water thus making a small change insignificant. Second would the the CaNo3 because calcium is also very abundant. Potassium on the other hand is used less by corals and probably the least abundant of the 3.
We can debate for years but randy Holmes Farley can tell you a definite answer in about 2 paragraphs. Before you dose anything I would ask his opinion.
We can debate for years but randy Holmes Farley can tell you a definite answer in about 2 paragraphs. Before you dose anything I would ask his opinion.
The most common I have read about is sodium nitrate. If I had to guess without looking its because sodium is probably one of the more abundant elements in salt water thus making a small change insignificant. Second would the the CaNo3 because calcium is also very abundant. Potassium on the other hand is used less by corals and probably the least abundant of the 3.
We can debate for years but randy Holmes Farley can tell you a definite answer in about 2 paragraphs. Before you dose anything I would ask his opinion.
The problem with using sodium nitrate would be the rise of salinity. We use calcium chloride for increasing or calcium. When the sodium and chloride combine it will make salt.
[...]randy Holmes Farley can tell you a definite answer in about 2 paragraphs. Before you dose anything I would ask his opinion.
Strange advice since he's not known to hang out here. Strange place to be if that's actually what you're looking for.
I like your reasoning on choosing which Nitrate to pick though...more or less the same reasoning for choosing the two-part ingredients we're all familiar with and why water changes are an integral part of two-part dosing. Na and Cl do slowly build up and need to be kept in some semblance of balance. Balling accounts for this a little differently than two-part, but they have to account for it too. That link is to a good vid that explains the Na+Cl issue pretty well.
Seems with the dosages that appear to be likely that this shouldn't make a huge difference though - especially if water changes are already part of the regime.
The problem with using sodium nitrate would be the rise of salinity. We use calcium chloride for increasing or calcium. When the sodium and chloride combine it will make salt.
All these things (and two-part as well) contribute to salinity. But unless you're adding a significant volume of material - e.g. hundreds of ppm per day - it shouldn't be very relevant.
Easy to keep an eye on with the refractometer.
-Matt
The problem with using sodium nitrate would be the rise of salinity. We use calcium chloride for increasing or calcium. When the sodium and chloride combine it will make salt.
But you dose so little to actually raise your Nitrate level that I don't believe the salinity would actually be affected that much. With the solution I provided above you dose 8.5ml per 120 gals 1ppm of Nitrate. Once its elevated to the desired level it shouldn't require a daily dose to maintain. Just every few days.
8.5ml per 120 gals TO RAISE LEVLES BY 1ppm of Nitrate
Similar threads
- Replies
- 8
- Views
- 256
- Replies
- 16
- Views
- 410
- Replies
- 8
- Views
- 480
- Replies
- 17
- Views
- 383
- Replies
- 16
- Views
- 291



















