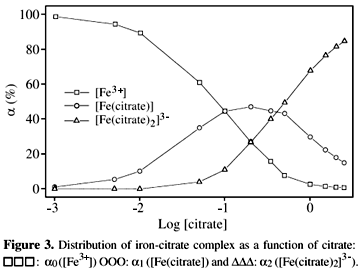
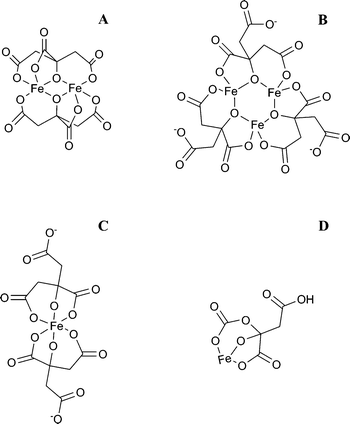
Hi Randy, having read your synthesis of iron citrate, I am unclear the type of sodium citrate required.
Wiki states that sodium citrate can exist in 3 forms, mono, di and tri sodium citrate. Mono sodium citrate is mentioned as being a strong sequestrant, but the common form of sodium citrate I can find on ebay is tri sodium citrate. I just wanted to confirm which form Randy uses in his iron citrate synt. I guess mono, but would like to confirm. Tri would be easier as theres no extra step (I can buy it rather than make it) but would it work?
I have sodium carbonate, citric acid (easily available), and can get ferrous sulphate on ebay, so I guess these starting chemicals are all thats required? (the only iron tabs I can find in the shops here contain lots of excipients like calcium phosphate and glycerine, in unknown concentrations, so I prefer to steer clear of them).
Thanks, Pete
Wiki states that sodium citrate can exist in 3 forms, mono, di and tri sodium citrate. Mono sodium citrate is mentioned as being a strong sequestrant, but the common form of sodium citrate I can find on ebay is tri sodium citrate. I just wanted to confirm which form Randy uses in his iron citrate synt. I guess mono, but would like to confirm. Tri would be easier as theres no extra step (I can buy it rather than make it) but would it work?
I have sodium carbonate, citric acid (easily available), and can get ferrous sulphate on ebay, so I guess these starting chemicals are all thats required? (the only iron tabs I can find in the shops here contain lots of excipients like calcium phosphate and glycerine, in unknown concentrations, so I prefer to steer clear of them).
Thanks, Pete