Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
How a Two Part Alkalinity and Calcium System Works, and Why it Matters
By Randy Holmes-Farley
This article is intended to help aquarists understand some of the important differences between the great variety of two and three part alkalinity and calcium dosing systems presently available, including commercial, DIY, and hybrids. It goes into detail about how such products are designed and what those design choices mean to a user.
While this article is not intended to be intensely technical, it is intended for medium to advanced aquarists, or those who want to really understand how products they use work. It is not intended to be a beginner’s guide to how to choose or use these sorts of products.
Sections in this article:
Why Calcium and Alkalinity
Basics of the Two Part Method
The Alkalinity Part
The Calcium Part
Ratio of the Two Parts
Accumulating Sodium and Chloride
Ion Balance
1. Incorporating some or all ions impacted by this issue into one of the two parts of a two part system.
2. A second method involves using a third part intended to correct these ion imbalances.
3. Ion Balance by water change
What about Ion Consumption Other than Calcium and Carbonate?
Commercial Two Parts with Extra Additives
Conclusions
Why Calcium and Alkalinity
There is no aspect of reef aquarium chemistry more important than calcium and alkalinity. Many of my previous articles have described various aspects of these systems in detail. In reading those articles, aquarists will note one pervasive theme: that maintaining appropriate levels of each are very important.
Moreover, the easiest way to ensure that things do not go seriously wrong in adding these to the aquarium is to use methods that have balanced amounts of calcium and alkalinity. In a previous article I detailed how many of these systems work. A balanced calcium and alkalinity additive is one that provides calcium and alkalinity in proportions that match that used by corals and other organisms to form calcium carbonate. Using this type of method typically prevents overdosing (or underdosing) of either of these two relative to the other and can allow for significantly less reliance on potentially inaccurate testing to maintain appropriate alkalinity and calcium.
Basics of the Two Part Method
One of the most popular of the balanced methods involve two (or three) parts comprising liquid additive solutions that are dosed at equal or otherwise mostly fixed ratios to maintain alkalinity and calcium, and possibly other ions. There are many different ways to implement two part systems, and each has different consequences for the aquarium. These systems include commercial products and DIY. Unfortunately, for reef aquarists, commercial systems often do not include enough information to know how they are designed and so one cannot know exactly what they will accomplish when used. These different possible ways of designing such products and what the implications are for the users are the primary focus of this article.
In general, a two or three part method includes one part that adds alkalinity, a second part that adds calcium, and may include a third part that attempts to deal with other ions in some fashion. What exactly is in each part varies tremendously, and is often among the poorly detailed aspects of commercial systems.

Figure 1. This wonderful aquarium by Reef2Reef member BobbyM used the ESV B-ionic two part alkalinity and calcium system.
The Alkalinity Part
The alkalinity part of any two part systems generally includes one or two of the following main ingredients:
Sodium bicarbonate
Sodium carbonate
Sodium hydroxide
The only functional difference between these components is the effect on aquarium pH, which also has implications for the tendency for local precipitation of calcium carbonate (will not redissolve) and magnesium hydroxide (will redissolve). All of them instantly add alkalinity detected by any testing method, so one can use them in automated (machine tests alkalinity and doses as needed to maintain a target level) semi-automated (aquarists determines a dose rate based on testing that is then used to set a dosing pump), or fully manual ways (reefer tests and doses by hand as needed).
The pH effect of each of these depends on the amount added, how fast it is added, what the alkalinity of the aquarium is at the start of the addition, and how much aeration the aquarium has. If one separately adds 1.4 dKH (0.5 meq/L) of each of these additives to artificial seawater at 6.3 dKH, the pH effect is approximately what is shown below.
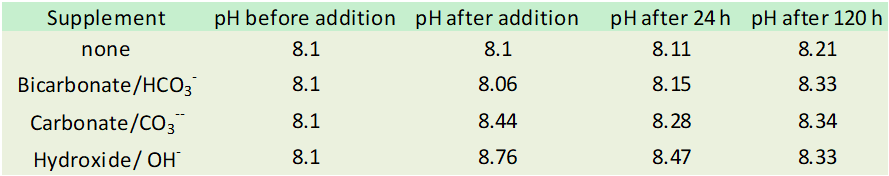
This experiment was performed in a beaker, not a reef tank, so the rate at which the pH values change after the initial addition will vary, but it shows several important aspects to such an addition:
1. The effects of a single addition are somewhat temporary (but of course, if you dose multiple times a day, every day, the effects will be sustained to some extent).
2. Bicarbonate has an initial pH lowering effect, albeit a small one.
3. Carbonate and hydroxide have substantial pH raising effects, with hydroxide having about twice the pH boost as carbonate. These reasons for all of these pH effects are well understood and I have discussed them in detail previously, but will not repeat the chemical equations involved here.
4. As the water equilibrates with the atmosphere, the pH of the different solutions converge on a value that is only dependent on the final alkalinity, and not at all on how it got there.
Thus, a very important aspect to the design of a two part is which additives are actually used for the alkalinity part, since that can have a substantial impact on the pH in the aquarium. In many situations, reef aquaria using only this method will often benefit from a pH boost due to the pH lowering effects of accumulating CO2 inside of many homes, so the best choices for most people will usually be a carbonate or hydroxide based alkalinity part. That said, there are both commercial and DIY additives using all of these, as well as mixtures of carbonate and bicarbonate. Hydroxide based systems are newer to the scene, and most current aquarists use a DIY version.
A second implication of the design choice between these additives is the solubility limit. At 20 °C, the solubility limits of these chemicals in fresh water are:
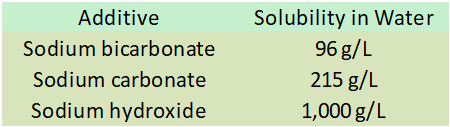
Thus, two part systems using sodium bicarbonate are typically about half as potent as those using sodium carbonate due to this solubility limit. Note that I do not recommend using sodium hydroxide at such high concentrations for a variety of reasons (local precipitation is increased, dosing control is harder, the solutions become more and more corrosive and a health risk to users, etc.). My DIY recipes using hydroxide are designed to match the potency of my DIY sodium carbonate recipes.
In some commercial and DIY systems, there is nothing else in the alkalinity part. Depending on the full recipe, that can be fine. However, in some systems (both commercial and DIY) there are other components in the alkalinity part that can be useful to balance the other ions in the system. This topic will be discussed in greater detail later in this article, but other ingredients in this part of a two part may include sodium sulfate, sodium bromide, potassium chloride, and more, to help balance out sulfate, bromide and potassium.

Figure 2. This nice aquarium from Reef2Reef member Olys_Tank used a three part from Fauna Marin (Balling Light).
The Calcium Part
Unlike the alkalinity part, there is little choice to be made on the calcium additives, and calcium chloride is the only suitable candidate. Others might be used in combination with calcium chloride, such as calcium bromide, but would be unnecessarily expensive and are probably not used to any significant extent. While one could use an organocalcium salt (calcium formate or calcium acetate, for example), that would provide both alkalinity and calcium and would make it a one part system that won’t be discussed here. Calcium chloride is highly soluble (about 750 g/L anhydrous calcium chloride dissolves in fresh water at 20 °C) so it is generally not a limitation in designing a two part, and can simply be matched to the chosen alkalinity potency.
Just like the alkalinity part, some commercial and DIY systems contain nothing else, and that can be fine. However, in some systems (both commercial and DIY) there are other components in the calcium part that can be useful to balance the other ions in the system. This topic will be discussed in greater detail later in this article, but other ingredients in this part of a two part may include magnesium chloride, sodium bromide, potassium chloride, and more, to help balance out magnesium, bromide and potassium.
Ratio of the Two Parts
The basic premise of most two part systems is to allow 1:1 dosing of the two parts to provide the alkalinity and calcium consumed by coral calcification and abiotic precipitation of calcium carbonate that may be happening in the aquarium.
The basic governing equations are these:
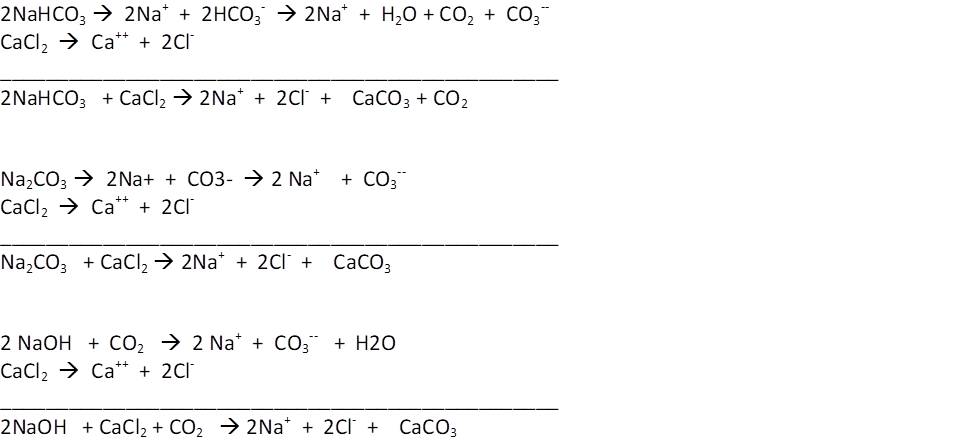
In each case, the critical aspect for designing a 1:1 two part is that it takes 2 units of alkalinity (2 NaOH or 2 NaHCO3 or one Na2CO3) for each unit of calcium. When designed in this fashion, the only residuals present in the water, aside from CO2 which can enter or leave to the air) are 2Na+ and 2 Cl-.
In the units of measure that reefkeepers most often use, that ratio is 2.8 dKH (alkalinity) for each 20 ppm of calcium. Thus the starting point for a two part recipe has that ratio between the two parts.
One complexity that some designers may take into account is that pure calcium carbonate does not form in a reef tank. Some of the calcium ions are replaced by magnesium, and a much smaller number by strontium, since they look very chemically like calcium and can slip into the crystal structure in its place. The strontium incorporation is small and mostly unchanged between organisms and abiotic precipitation, but magnesium incorporation varies substantially, from almost none in some corals to about 1/10th of the calcium mass being replaced by magnesium by coralline algae.
A consequence of this incorporation of Mg and Sr is that the amount of calcium incorporated will be less than exactly 20 ppm for each 2.8 dKH of alkalinity consumed by calcification. Reef tank consumption is about 18-20 ppm calcium and 0-2 ppm magnesium to 2.8 dKH alkalinity. Some recipes may take that difference into account and some may not. There’s nothing wrong with either way, and but to do it, one has to make assumptions about how much magnesium gets incorporated on average. The long term effects will be different with different assumptions made, and a recipe that does not take it into account at all may cause calcium to slowly rise over time if the method is used to replace all consumed alkalinity. That effect does not show up in a week or two, but will show up after many weeks to months (if not lost in the noise of ongoing water changes). Curiously, Seachem Reef Fusion claims to be 1:1 dosing but the recipe incorporates 2.46 dKH of alkalinity for each 20 ppm of calcium. I’m not sure why they elected that ratio, but it seems to contain substantially excess calcium.

Figure 3. This reef aquarium by reef2reef member Mickeyt1reef uses a combination of one of my DIY recipes (soda ash and calcium chloride sold by BRS) along with kalkwasser for calcium and alkalinity. The magnesium/part 3 is only dosed as needed. In general, it is fine to mix and match calcium and alkalinity dosing methods.
Accumulating Sodium and Chloride
A very important consequence of using a two part is that sodium and chloride are added with every dose. Over time, this effect is quite significant. The actual salinity increase will vary based on exactly what other ingredients are incorporated into the two part, and in what form, but we can estimate the minimum effect one would see without water changes from just the alkalinity and calcium additions.
Assumption: add 1 dKH of alkalinity and the balanced amount of 7 ppm calcium each day. 1 dKH of alkalinity causes 0.36 meq/L of sodium to accumulate, or 8 mg/L sodium. The calcium chloride addition will add 12.4 ppm of chloride. Importantly, the Na to Cl ratio is exactly that found in NaCl, and we will come back to that later. We are thus adding 8 + 12.4 mg/l every day, or 632 mg/L in a month = 0.63 ppt each month =7.5 ppt in a year. Note that this is a minimum effect and does not count additions of other ions that may be present, such as sulfate.
This salinity rise means that aquarists may have to offset that rise by removing some salt water periodically and replacing it with fresh. It may happen partly by skimming, but will also likely require some intervention. Do not rely on the above number for intervention purposes because some two parts may contain much more material (e.g., sulfate, etc.). Make the changes as you would for any other factor impacting salinity: by testing and adjusting when needed).
Further, when one lowers the salinity to offset the rise from the accumulating sodium and chloride, one is necessarily depressing every other ion in the water. For example, if you dose at the 1 dKH per day rate, do no water changes, then after a year drop the salinity by 7.5 ppt, the concentration of every ion in the water will drop by a factor of (35-7.5 ppt)/(35 ppt) to 79% of its value on the first of the year. Potassium, for example, would drop from 400 ppm to 316 pm. Even worse, magnesium is not only depressed by this factor (1300 ppm to 1027 ppm) but was also getting consumed along the way.
Water changes will reduce these problems, but most two part systems try to deal with them directly in any of several ways that are discussed in the next section.

Figure 4. This reef aquarium maintained by reef2reef member Phil D. uses the 1-2-3 two part made by Aquaforest.
Ion Balance
There are three fundamentally different ways to dealing with the ion imbalances and reductions caused by the accumulating sodium and chloride and subsequent salinity adjustments. These are:
1. Incorporating some or all ions impacted by this issue into one of the two parts of a two part system. This method is used by ESV B-ionic and a number of other brands. I have never seen any data of any sort that shows how well any manufacturer implements this method, and to elect a commercial system of this sort requires a substantial amount of trust that they know what they are doing, and then also did it properly. There are also some DIY recipes that use this method, though they are typically limited in the number of ions used.
This overall procedure involves more than just adding what was taken out in the salinity correction because sodium and chloride actually rise more than the salinity overall. In our example above, salinity rose by 7.5 ppt (21%) over a year, while sodium rose by 8 mg/L per day = 2920 mg/L in a year, which is approximately 10,800 mg/L to 13720 mg/L, or a 27% rise. Thus, even if we correct salinity back by 21%, sodium will still be high and everything else (except chloride) will be a bit low. To keep the water looking like seawater, one needs to add back even more of the ions that were depressed, which in turn raises salinity again, which in turn needs another correction. One can solve this problem on a spread sheet, and I won’t go into the complexity. That complexity, however, is a reason to only use a two part where you have confidence that the employees of the company are able to understand and deal with that complexity in design.
The other aspect of this design is deciding what forms of the ions can be put into which part of the two part. Sulfate is the biggest mass additive in this procedure, and it must be put into the alkalinity part because putting it into the calcium part results in precipitation of calcium sulfate. The designers also need to decide how to add it. It is likely all sodium sulfate, (boosting sodium again) or it could partly be potassium sulfate and other ions. In short, it’s a logic puzzle for chemists to decide what can be put where for the lowest cost and best performance. Since there are potentially dozens of chemicals to worry about, it is a nontrivial problem.
2. A second method involves using a third part intended to correct these ion imbalances. This method is technically simpler than #1 since one is not deciding what can go where.
Commercial approaches in this regard include Tropic Marin Balling Part C and Aquaforest Reef Mineral Salt as the third part. These are artificial sea salt mixes without the sodium chloride, and are seemingly easy for a salt mix manufacturer to make correctly. The reason such a system can work is that, as noted above, the accumulating sodium and chloride are in the same ratio as in sodium chloride. Sodium chloride is the biggest ingredient in any salt mix (it must be), but if it is left out, and combined with the accumulating sodium chloride from use of the alkalinity and calcium parts, one can have a residue that matches the original salt mix in all aspects.
These third parts work equally well whether the alkalinity part is sodium bicarbonate, or carbonate or hydroxide, since all add the same amount of sodium per unit of alkalinity added. They are thus easily combined with any other two part, such as a DIY, to take a hybrid approach to save costs or to use sodium hydroxide for the alkalinity part.
One minor point is that while conceptually this works out well, if one is doing water changes along the way, between when the excess sodium and chloride were added and when the third part is added and salinity is corrected, the final effect will not be perfect. I think this effect is quite minor unless one waits a long time before making the adjustments. Ideally, one would frequently be adding this third part. The amount to use will depend on how much of the other two parts are being used.
Another issue is that use of sodium chloride free salt cannot offset ANY consumption of ions, such as magnesium or trace elements, unless it is not actually sodium chloride free salt as both companies claim, and those ions may need to be added in some other fashion.
Finally, it is not clear to me whether these mixtures actually contain calcium or alkalinity. If they do, as they seeming claim from the description, that may limit how concentrated they can be made for dosing (due to calcium sulfate and carbonate precipitation), but my guess is they leave them out without telling folks. That issue does not really concern a user either way since they will be dosing and controlling calcium and alkalinity anyway.
There are DIY recipes for this part 3, and my DIY two part recipes do include a third part. In my recipe, this third part is primarily designed to deal with magnesium and sulfate depletion, and is not focused on many other ions (such as bromide). They are cheaper, and many users have shown them adequate over the many years they have been used, especially when also doing water changes, but they are clearly less complete than the sodium chloride free salt mixes described above.
3. Ion balance by water change. A third approach is to use just the alkalinity and calcium parts described above (with no additional ions in them) and to rely on water changes to make up any corrections. This approach is clearly the least expensive for the additives, but may be substantial in costs for water changes. Many years ago, Craig Bingman modelled the effects of this system in two articles (#1 and #2)and came to this conclusion:
“These simulations show that at low calcification rates and with what many people would consider to be substantial water exchange rates, calcium chloride and sodium bicarbonate can be used to meet the necessary calcium carbonate demand in a mixed aquarium containing a few hard corals, some live rock and live sand, without totally wrecking the composition of the water in the tank.”

Figure 5. This nice reef aquarium by Reef2Reef member LobsterOfJustice used method 3: just calcium chloride, sodium carbonate, a little magnesium chloride, and water changes.
What about Ion Consumption Other than Calcium and Carbonate?
A final aspect of the design of a two part is whether it is intended to compensate for any consumption of any other ions. Magnesium, for example. Is it only present there to offset the salinity increase issue (as the sodium chloride free salt would do), or to offset consumption in addition to dealing with the salinity increase?
In my DIY recipe, I do both for magnesium, with about 1/3 of the magnesium added to offset consumption and 2/3 used to offset the salinity rise issues. That same question can apply to trace elements as well, since they are also rapidly consumed in some cases.
However, not all two parts claim to offset ANY consumption of any ions except calcium and carbonate, and assuming they do should not be the default even if they say they contain all sorts of ions. This complexity has led many people to falsely assume the two part they are using will boost a trace element because it is present. That is not necessarily so. ESV B-ionic and Tropic Marin Balling clearly meet this situation: they add many elements, but do not offset any consumption.
Here’s a related example that has confused many reef aquarists relating to the presence of trace elements. Assuming that these products are actually formulated with every ion such that a true natural seawater residue remained (let’s call this the “ideal” product), then it will necessarily contain such ions as copper. Since copper may be elevated in some reef tanks, and is toxic to many invertebrates, reef keepers have sometimes wrongly criticized this method as adding more copper. That’s actually not what would happen. Since these products leave a natural seawater residue, and since copper may be elevated in concentration in some reef tanks relative to seawater, then using these “ideal” products will actually LOWER copper levels because when the increase in salinity is corrected, the copper will drop.
Here’s how that can work out:
You have copper in your aquarium at 4 ppb and salinity of S=35.
You add a two part additive (with Part 3 if that is the style) that over the course of a month raises salinity to S=36, and raises copper to 4.02 ppb.
Then you correct the salinity back to S=35 by diluting everything in the tank with fresh water, and you get a final copper concentration of 3.9 ppb. The fact that the product contained copper did not cause it to rise after using the product.
Does this happen in real products and not “ideal” products? I have no idea. But the statement by manufacturers that it contains all ions in natural ratios, including copper, should not be viewed as a concern that it is exacerbating a heavy metal problem, nor should it be viewed as a way to supplement these ions.
In fact, the effect these two parts have on magnesium and various trace elements is best thought of as a tiny water change each day. Perhaps on the order of 0.05 to 0.1% per day (2-3% per month).
Commercial Two Parts with Extra Additives
Of course, there’s no inherent reason that one cannot design a two part with extra additives to offset consumption. It can be hard to tell what a manufacturer is doing if all they say is it contains trace elements or some such thing, but others do clearly seem to indicate that they add more (but never how much). Aquaforest, for example, says
Chemical composition of Component 1+2+3+ is based on the method developed by H. Balling. The formula has been fine tuned in our lab and enriched with trace elements that are fundamental for maintaining marine aquarium organisms in good form.
As mentioned above, there’s an element of trust in what you use, since there’s no data on what these products contain aside from the alkalinity and calcium, but you can back up that trust up by measurement of ions that you are most concerned with (say, by ICP).
Conclusions
As should be clear from this discussion, two part systems may be much more complicated and have more different design criteria than may be apparent to users. Understanding what these various design possibilities are, and how different products implement them should help aquarists to choose products that best fit their goals. There is no single best way to implement these sort of products, so I leave it to each reader to decide for themselves which attributes of these systems best fit their own needs, and which companies they trust to properly attain those attributes.
Happy Reefing!
By Randy Holmes-Farley
This article is intended to help aquarists understand some of the important differences between the great variety of two and three part alkalinity and calcium dosing systems presently available, including commercial, DIY, and hybrids. It goes into detail about how such products are designed and what those design choices mean to a user.
While this article is not intended to be intensely technical, it is intended for medium to advanced aquarists, or those who want to really understand how products they use work. It is not intended to be a beginner’s guide to how to choose or use these sorts of products.
Sections in this article:
Why Calcium and Alkalinity
Basics of the Two Part Method
The Alkalinity Part
The Calcium Part
Ratio of the Two Parts
Accumulating Sodium and Chloride
Ion Balance
1. Incorporating some or all ions impacted by this issue into one of the two parts of a two part system.
2. A second method involves using a third part intended to correct these ion imbalances.
3. Ion Balance by water change
What about Ion Consumption Other than Calcium and Carbonate?
Commercial Two Parts with Extra Additives
Conclusions
Why Calcium and Alkalinity
There is no aspect of reef aquarium chemistry more important than calcium and alkalinity. Many of my previous articles have described various aspects of these systems in detail. In reading those articles, aquarists will note one pervasive theme: that maintaining appropriate levels of each are very important.
Moreover, the easiest way to ensure that things do not go seriously wrong in adding these to the aquarium is to use methods that have balanced amounts of calcium and alkalinity. In a previous article I detailed how many of these systems work. A balanced calcium and alkalinity additive is one that provides calcium and alkalinity in proportions that match that used by corals and other organisms to form calcium carbonate. Using this type of method typically prevents overdosing (or underdosing) of either of these two relative to the other and can allow for significantly less reliance on potentially inaccurate testing to maintain appropriate alkalinity and calcium.
Basics of the Two Part Method
One of the most popular of the balanced methods involve two (or three) parts comprising liquid additive solutions that are dosed at equal or otherwise mostly fixed ratios to maintain alkalinity and calcium, and possibly other ions. There are many different ways to implement two part systems, and each has different consequences for the aquarium. These systems include commercial products and DIY. Unfortunately, for reef aquarists, commercial systems often do not include enough information to know how they are designed and so one cannot know exactly what they will accomplish when used. These different possible ways of designing such products and what the implications are for the users are the primary focus of this article.
In general, a two or three part method includes one part that adds alkalinity, a second part that adds calcium, and may include a third part that attempts to deal with other ions in some fashion. What exactly is in each part varies tremendously, and is often among the poorly detailed aspects of commercial systems.
Figure 1. This wonderful aquarium by Reef2Reef member BobbyM used the ESV B-ionic two part alkalinity and calcium system.
The Alkalinity Part
The alkalinity part of any two part systems generally includes one or two of the following main ingredients:
Sodium bicarbonate
Sodium carbonate
Sodium hydroxide
The only functional difference between these components is the effect on aquarium pH, which also has implications for the tendency for local precipitation of calcium carbonate (will not redissolve) and magnesium hydroxide (will redissolve). All of them instantly add alkalinity detected by any testing method, so one can use them in automated (machine tests alkalinity and doses as needed to maintain a target level) semi-automated (aquarists determines a dose rate based on testing that is then used to set a dosing pump), or fully manual ways (reefer tests and doses by hand as needed).
The pH effect of each of these depends on the amount added, how fast it is added, what the alkalinity of the aquarium is at the start of the addition, and how much aeration the aquarium has. If one separately adds 1.4 dKH (0.5 meq/L) of each of these additives to artificial seawater at 6.3 dKH, the pH effect is approximately what is shown below.
This experiment was performed in a beaker, not a reef tank, so the rate at which the pH values change after the initial addition will vary, but it shows several important aspects to such an addition:
1. The effects of a single addition are somewhat temporary (but of course, if you dose multiple times a day, every day, the effects will be sustained to some extent).
2. Bicarbonate has an initial pH lowering effect, albeit a small one.
3. Carbonate and hydroxide have substantial pH raising effects, with hydroxide having about twice the pH boost as carbonate. These reasons for all of these pH effects are well understood and I have discussed them in detail previously, but will not repeat the chemical equations involved here.
4. As the water equilibrates with the atmosphere, the pH of the different solutions converge on a value that is only dependent on the final alkalinity, and not at all on how it got there.
Thus, a very important aspect to the design of a two part is which additives are actually used for the alkalinity part, since that can have a substantial impact on the pH in the aquarium. In many situations, reef aquaria using only this method will often benefit from a pH boost due to the pH lowering effects of accumulating CO2 inside of many homes, so the best choices for most people will usually be a carbonate or hydroxide based alkalinity part. That said, there are both commercial and DIY additives using all of these, as well as mixtures of carbonate and bicarbonate. Hydroxide based systems are newer to the scene, and most current aquarists use a DIY version.
A second implication of the design choice between these additives is the solubility limit. At 20 °C, the solubility limits of these chemicals in fresh water are:
Thus, two part systems using sodium bicarbonate are typically about half as potent as those using sodium carbonate due to this solubility limit. Note that I do not recommend using sodium hydroxide at such high concentrations for a variety of reasons (local precipitation is increased, dosing control is harder, the solutions become more and more corrosive and a health risk to users, etc.). My DIY recipes using hydroxide are designed to match the potency of my DIY sodium carbonate recipes.
In some commercial and DIY systems, there is nothing else in the alkalinity part. Depending on the full recipe, that can be fine. However, in some systems (both commercial and DIY) there are other components in the alkalinity part that can be useful to balance the other ions in the system. This topic will be discussed in greater detail later in this article, but other ingredients in this part of a two part may include sodium sulfate, sodium bromide, potassium chloride, and more, to help balance out sulfate, bromide and potassium.
Figure 2. This nice aquarium from Reef2Reef member Olys_Tank used a three part from Fauna Marin (Balling Light).
The Calcium Part
Unlike the alkalinity part, there is little choice to be made on the calcium additives, and calcium chloride is the only suitable candidate. Others might be used in combination with calcium chloride, such as calcium bromide, but would be unnecessarily expensive and are probably not used to any significant extent. While one could use an organocalcium salt (calcium formate or calcium acetate, for example), that would provide both alkalinity and calcium and would make it a one part system that won’t be discussed here. Calcium chloride is highly soluble (about 750 g/L anhydrous calcium chloride dissolves in fresh water at 20 °C) so it is generally not a limitation in designing a two part, and can simply be matched to the chosen alkalinity potency.
Just like the alkalinity part, some commercial and DIY systems contain nothing else, and that can be fine. However, in some systems (both commercial and DIY) there are other components in the calcium part that can be useful to balance the other ions in the system. This topic will be discussed in greater detail later in this article, but other ingredients in this part of a two part may include magnesium chloride, sodium bromide, potassium chloride, and more, to help balance out magnesium, bromide and potassium.
Ratio of the Two Parts
The basic premise of most two part systems is to allow 1:1 dosing of the two parts to provide the alkalinity and calcium consumed by coral calcification and abiotic precipitation of calcium carbonate that may be happening in the aquarium.
The basic governing equations are these:
In each case, the critical aspect for designing a 1:1 two part is that it takes 2 units of alkalinity (2 NaOH or 2 NaHCO3 or one Na2CO3) for each unit of calcium. When designed in this fashion, the only residuals present in the water, aside from CO2 which can enter or leave to the air) are 2Na+ and 2 Cl-.
In the units of measure that reefkeepers most often use, that ratio is 2.8 dKH (alkalinity) for each 20 ppm of calcium. Thus the starting point for a two part recipe has that ratio between the two parts.
One complexity that some designers may take into account is that pure calcium carbonate does not form in a reef tank. Some of the calcium ions are replaced by magnesium, and a much smaller number by strontium, since they look very chemically like calcium and can slip into the crystal structure in its place. The strontium incorporation is small and mostly unchanged between organisms and abiotic precipitation, but magnesium incorporation varies substantially, from almost none in some corals to about 1/10th of the calcium mass being replaced by magnesium by coralline algae.
A consequence of this incorporation of Mg and Sr is that the amount of calcium incorporated will be less than exactly 20 ppm for each 2.8 dKH of alkalinity consumed by calcification. Reef tank consumption is about 18-20 ppm calcium and 0-2 ppm magnesium to 2.8 dKH alkalinity. Some recipes may take that difference into account and some may not. There’s nothing wrong with either way, and but to do it, one has to make assumptions about how much magnesium gets incorporated on average. The long term effects will be different with different assumptions made, and a recipe that does not take it into account at all may cause calcium to slowly rise over time if the method is used to replace all consumed alkalinity. That effect does not show up in a week or two, but will show up after many weeks to months (if not lost in the noise of ongoing water changes). Curiously, Seachem Reef Fusion claims to be 1:1 dosing but the recipe incorporates 2.46 dKH of alkalinity for each 20 ppm of calcium. I’m not sure why they elected that ratio, but it seems to contain substantially excess calcium.
Figure 3. This reef aquarium by reef2reef member Mickeyt1reef uses a combination of one of my DIY recipes (soda ash and calcium chloride sold by BRS) along with kalkwasser for calcium and alkalinity. The magnesium/part 3 is only dosed as needed. In general, it is fine to mix and match calcium and alkalinity dosing methods.
Accumulating Sodium and Chloride
A very important consequence of using a two part is that sodium and chloride are added with every dose. Over time, this effect is quite significant. The actual salinity increase will vary based on exactly what other ingredients are incorporated into the two part, and in what form, but we can estimate the minimum effect one would see without water changes from just the alkalinity and calcium additions.
Assumption: add 1 dKH of alkalinity and the balanced amount of 7 ppm calcium each day. 1 dKH of alkalinity causes 0.36 meq/L of sodium to accumulate, or 8 mg/L sodium. The calcium chloride addition will add 12.4 ppm of chloride. Importantly, the Na to Cl ratio is exactly that found in NaCl, and we will come back to that later. We are thus adding 8 + 12.4 mg/l every day, or 632 mg/L in a month = 0.63 ppt each month =7.5 ppt in a year. Note that this is a minimum effect and does not count additions of other ions that may be present, such as sulfate.
This salinity rise means that aquarists may have to offset that rise by removing some salt water periodically and replacing it with fresh. It may happen partly by skimming, but will also likely require some intervention. Do not rely on the above number for intervention purposes because some two parts may contain much more material (e.g., sulfate, etc.). Make the changes as you would for any other factor impacting salinity: by testing and adjusting when needed).
Further, when one lowers the salinity to offset the rise from the accumulating sodium and chloride, one is necessarily depressing every other ion in the water. For example, if you dose at the 1 dKH per day rate, do no water changes, then after a year drop the salinity by 7.5 ppt, the concentration of every ion in the water will drop by a factor of (35-7.5 ppt)/(35 ppt) to 79% of its value on the first of the year. Potassium, for example, would drop from 400 ppm to 316 pm. Even worse, magnesium is not only depressed by this factor (1300 ppm to 1027 ppm) but was also getting consumed along the way.
Water changes will reduce these problems, but most two part systems try to deal with them directly in any of several ways that are discussed in the next section.
Figure 4. This reef aquarium maintained by reef2reef member Phil D. uses the 1-2-3 two part made by Aquaforest.
Ion Balance
There are three fundamentally different ways to dealing with the ion imbalances and reductions caused by the accumulating sodium and chloride and subsequent salinity adjustments. These are:
1. Incorporating some or all ions impacted by this issue into one of the two parts of a two part system. This method is used by ESV B-ionic and a number of other brands. I have never seen any data of any sort that shows how well any manufacturer implements this method, and to elect a commercial system of this sort requires a substantial amount of trust that they know what they are doing, and then also did it properly. There are also some DIY recipes that use this method, though they are typically limited in the number of ions used.
This overall procedure involves more than just adding what was taken out in the salinity correction because sodium and chloride actually rise more than the salinity overall. In our example above, salinity rose by 7.5 ppt (21%) over a year, while sodium rose by 8 mg/L per day = 2920 mg/L in a year, which is approximately 10,800 mg/L to 13720 mg/L, or a 27% rise. Thus, even if we correct salinity back by 21%, sodium will still be high and everything else (except chloride) will be a bit low. To keep the water looking like seawater, one needs to add back even more of the ions that were depressed, which in turn raises salinity again, which in turn needs another correction. One can solve this problem on a spread sheet, and I won’t go into the complexity. That complexity, however, is a reason to only use a two part where you have confidence that the employees of the company are able to understand and deal with that complexity in design.
The other aspect of this design is deciding what forms of the ions can be put into which part of the two part. Sulfate is the biggest mass additive in this procedure, and it must be put into the alkalinity part because putting it into the calcium part results in precipitation of calcium sulfate. The designers also need to decide how to add it. It is likely all sodium sulfate, (boosting sodium again) or it could partly be potassium sulfate and other ions. In short, it’s a logic puzzle for chemists to decide what can be put where for the lowest cost and best performance. Since there are potentially dozens of chemicals to worry about, it is a nontrivial problem.
2. A second method involves using a third part intended to correct these ion imbalances. This method is technically simpler than #1 since one is not deciding what can go where.
Commercial approaches in this regard include Tropic Marin Balling Part C and Aquaforest Reef Mineral Salt as the third part. These are artificial sea salt mixes without the sodium chloride, and are seemingly easy for a salt mix manufacturer to make correctly. The reason such a system can work is that, as noted above, the accumulating sodium and chloride are in the same ratio as in sodium chloride. Sodium chloride is the biggest ingredient in any salt mix (it must be), but if it is left out, and combined with the accumulating sodium chloride from use of the alkalinity and calcium parts, one can have a residue that matches the original salt mix in all aspects.
These third parts work equally well whether the alkalinity part is sodium bicarbonate, or carbonate or hydroxide, since all add the same amount of sodium per unit of alkalinity added. They are thus easily combined with any other two part, such as a DIY, to take a hybrid approach to save costs or to use sodium hydroxide for the alkalinity part.
One minor point is that while conceptually this works out well, if one is doing water changes along the way, between when the excess sodium and chloride were added and when the third part is added and salinity is corrected, the final effect will not be perfect. I think this effect is quite minor unless one waits a long time before making the adjustments. Ideally, one would frequently be adding this third part. The amount to use will depend on how much of the other two parts are being used.
Another issue is that use of sodium chloride free salt cannot offset ANY consumption of ions, such as magnesium or trace elements, unless it is not actually sodium chloride free salt as both companies claim, and those ions may need to be added in some other fashion.
Finally, it is not clear to me whether these mixtures actually contain calcium or alkalinity. If they do, as they seeming claim from the description, that may limit how concentrated they can be made for dosing (due to calcium sulfate and carbonate precipitation), but my guess is they leave them out without telling folks. That issue does not really concern a user either way since they will be dosing and controlling calcium and alkalinity anyway.
There are DIY recipes for this part 3, and my DIY two part recipes do include a third part. In my recipe, this third part is primarily designed to deal with magnesium and sulfate depletion, and is not focused on many other ions (such as bromide). They are cheaper, and many users have shown them adequate over the many years they have been used, especially when also doing water changes, but they are clearly less complete than the sodium chloride free salt mixes described above.
3. Ion balance by water change. A third approach is to use just the alkalinity and calcium parts described above (with no additional ions in them) and to rely on water changes to make up any corrections. This approach is clearly the least expensive for the additives, but may be substantial in costs for water changes. Many years ago, Craig Bingman modelled the effects of this system in two articles (#1 and #2)and came to this conclusion:
“These simulations show that at low calcification rates and with what many people would consider to be substantial water exchange rates, calcium chloride and sodium bicarbonate can be used to meet the necessary calcium carbonate demand in a mixed aquarium containing a few hard corals, some live rock and live sand, without totally wrecking the composition of the water in the tank.”
Figure 5. This nice reef aquarium by Reef2Reef member LobsterOfJustice used method 3: just calcium chloride, sodium carbonate, a little magnesium chloride, and water changes.
What about Ion Consumption Other than Calcium and Carbonate?
A final aspect of the design of a two part is whether it is intended to compensate for any consumption of any other ions. Magnesium, for example. Is it only present there to offset the salinity increase issue (as the sodium chloride free salt would do), or to offset consumption in addition to dealing with the salinity increase?
In my DIY recipe, I do both for magnesium, with about 1/3 of the magnesium added to offset consumption and 2/3 used to offset the salinity rise issues. That same question can apply to trace elements as well, since they are also rapidly consumed in some cases.
However, not all two parts claim to offset ANY consumption of any ions except calcium and carbonate, and assuming they do should not be the default even if they say they contain all sorts of ions. This complexity has led many people to falsely assume the two part they are using will boost a trace element because it is present. That is not necessarily so. ESV B-ionic and Tropic Marin Balling clearly meet this situation: they add many elements, but do not offset any consumption.
Here’s a related example that has confused many reef aquarists relating to the presence of trace elements. Assuming that these products are actually formulated with every ion such that a true natural seawater residue remained (let’s call this the “ideal” product), then it will necessarily contain such ions as copper. Since copper may be elevated in some reef tanks, and is toxic to many invertebrates, reef keepers have sometimes wrongly criticized this method as adding more copper. That’s actually not what would happen. Since these products leave a natural seawater residue, and since copper may be elevated in concentration in some reef tanks relative to seawater, then using these “ideal” products will actually LOWER copper levels because when the increase in salinity is corrected, the copper will drop.
Here’s how that can work out:
You have copper in your aquarium at 4 ppb and salinity of S=35.
You add a two part additive (with Part 3 if that is the style) that over the course of a month raises salinity to S=36, and raises copper to 4.02 ppb.
Then you correct the salinity back to S=35 by diluting everything in the tank with fresh water, and you get a final copper concentration of 3.9 ppb. The fact that the product contained copper did not cause it to rise after using the product.
Does this happen in real products and not “ideal” products? I have no idea. But the statement by manufacturers that it contains all ions in natural ratios, including copper, should not be viewed as a concern that it is exacerbating a heavy metal problem, nor should it be viewed as a way to supplement these ions.
In fact, the effect these two parts have on magnesium and various trace elements is best thought of as a tiny water change each day. Perhaps on the order of 0.05 to 0.1% per day (2-3% per month).
Commercial Two Parts with Extra Additives
Of course, there’s no inherent reason that one cannot design a two part with extra additives to offset consumption. It can be hard to tell what a manufacturer is doing if all they say is it contains trace elements or some such thing, but others do clearly seem to indicate that they add more (but never how much). Aquaforest, for example, says
Chemical composition of Component 1+2+3+ is based on the method developed by H. Balling. The formula has been fine tuned in our lab and enriched with trace elements that are fundamental for maintaining marine aquarium organisms in good form.
As mentioned above, there’s an element of trust in what you use, since there’s no data on what these products contain aside from the alkalinity and calcium, but you can back up that trust up by measurement of ions that you are most concerned with (say, by ICP).
Conclusions
As should be clear from this discussion, two part systems may be much more complicated and have more different design criteria than may be apparent to users. Understanding what these various design possibilities are, and how different products implement them should help aquarists to choose products that best fit their goals. There is no single best way to implement these sort of products, so I leave it to each reader to decide for themselves which attributes of these systems best fit their own needs, and which companies they trust to properly attain those attributes.
Happy Reefing!













