Thanks - Just what hoped to hearThose values are close enough that I would not be concerned about the mismatch at all.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
How a Two Part Alkalinity and Calcium System Works, and Why it Matters
- Thread starter Randy Holmes-Farley
- Start date
- Tagged users None
Randy, this chart
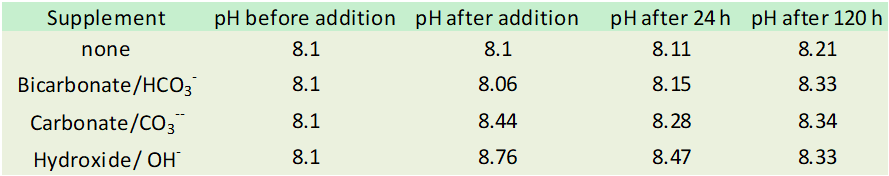
The first row (none) , why is there increase in pH after 120 hours? Nothing was added… Is that just variation in CO2 in the air?
Also I am bit confused with this comment:

Mainly the calcium carbonate precipitation. Does that also mean that other trace elements and perhaps other compounds also precipitate out? I think that might be somewhat of a negative in using high pH system.
The reason I am asking or am curious is that for 5 years I used bicarbonate or AFR (low pH additives) and had no calcium build up. Once I switched to Hydroxide oh boy the skimmer pump efficiency dropped and when I pulled it apart there was calcium buildup that I have not seen and same for my return pump.
Based on my limited experience (one tank) the high pH additives seem to have some negatives that are not really mentioned. Also I suspect the precipitate will accumulate over time? This leads to more maintenance and additional dosing due to the precipitation.
I find the low pH additives gentler on my tank. Made for time challenged people/reefers like me.
The first row (none) , why is there increase in pH after 120 hours? Nothing was added… Is that just variation in CO2 in the air?
Also I am bit confused with this comment:
Mainly the calcium carbonate precipitation. Does that also mean that other trace elements and perhaps other compounds also precipitate out? I think that might be somewhat of a negative in using high pH system.
The reason I am asking or am curious is that for 5 years I used bicarbonate or AFR (low pH additives) and had no calcium build up. Once I switched to Hydroxide oh boy the skimmer pump efficiency dropped and when I pulled it apart there was calcium buildup that I have not seen and same for my return pump.
Based on my limited experience (one tank) the high pH additives seem to have some negatives that are not really mentioned. Also I suspect the precipitate will accumulate over time? This leads to more maintenance and additional dosing due to the precipitation.
I find the low pH additives gentler on my tank. Made for time challenged people/reefers like me.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
The first row (none) , why is there increase in pH after 120 hours? Nothing was added… Is that just variation in CO2 in the air?
Yes, it had some excess CO2 initially, and equilibrated with the air in that room (a lab with high air flow so pretty much outside air).
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
Randy, this chart
Mainly the calcium carbonate precipitation. Does that also mean that other trace elements and perhaps other compounds also precipitate out? I think that might be somewhat of a negative in using high pH system.
The reason I am asking or am curious is that for 5 years I used bicarbonate or AFR (low pH additives) and had no calcium build up. Once I switched to Hydroxide oh boy the skimmer pump efficiency dropped and when I pulled it apart there was calcium buildup that I have not seen and same for my return pump.
I expect that any time calcium carbonate surfaces are present, either from sand/rock or from new precipitation, some elements and molecules bind to those surfaces.
But the locally high pH does not necessarily mean that trace elements are precipitating on their own.
IMO, if you want to dose very high pH additives, it is best to not do so right near equipment that it might precipitate onto.
Why are you using those concentrations? It does not seem designed for 1:1 dosing.
My recommended recipe is:
Metoda Ballinga - Metoda suplementacji minerałów
Metoda Ballinga - w artykule wyjaśniamy podstawy suplementacji minerałów oraz wyjaśniamy procesy chemiczne rządzące tą metodą.
I use the concentrations recommended on the Polish website. Calcium in anhydrous form, therefore 55.5g/liter. Instead of NaHCO3 in the recommended amount of 84g/liter, I used NaOH in the amount of 40g/liter, because this conversion rate was recommended. From what I quickly calculated, the concentration ratio of your recipe and the one I used is only slightly different and to make it match, I should dissolve NaOH in the amount of 41.7g/liter.
I hope I calculated correctly and used the appropriate gallon to liter conversion rate (1 gallon = 3.785 liters).
Do I think right?
Last edited:
Hi Reef Chemist,
Great information! What do you think of careful two-part dosing of selected wild reefs during peak acidification events? Is it worthwhile?
Many propose "Ocean Alkalinity Enhancement" (OAE) but lack the practical and exact scientific knowledge of the unique reefer community.
Cheers!
Great information! What do you think of careful two-part dosing of selected wild reefs during peak acidification events? Is it worthwhile?
Many propose "Ocean Alkalinity Enhancement" (OAE) but lack the practical and exact scientific knowledge of the unique reefer community.
Cheers!
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
Hi Reef Chemist,
Great information! What do you think of careful two-part dosing of selected wild reefs during peak acidification events? Is it worthwhile?
Many propose "Ocean Alkalinity Enhancement" (OAE) but lack the practical and exact scientific knowledge of the unique reefer community.
Cheers!
IMO, it's technically feasible but too costly to actually get implemented on a useful scale. It would require extensive infrastructure to add it in a way that did not boost anything too excessively at any given location.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827

Metoda Ballinga - Metoda suplementacji minerałów
Metoda Ballinga - w artykule wyjaśniamy podstawy suplementacji minerałów oraz wyjaśniamy procesy chemiczne rządzące tą metodą.reefhub.pl
I use the concentrations recommended on the Polish website. Calcium in anhydrous form, therefore 55.5g/liter. Instead of NaHCO3 in the recommended amount of 84g/liter, I used NaOH in the amount of 40g/liter, because this conversion rate was recommended. From what I quickly calculated, the concentration ratio of your recipe and the one I used is only slightly different and to make it match, I should dissolve NaOH in the amount of 41.7g/liter.
I hope I calculated correctly and used the appropriate gallon to liter conversion rate (1 gallon = 3.785 liters).
Do I think right?
OK, that ratio is fine since you are using anhydrous calcium chloride.
Thr article indicates “ so the best choices for most people will usually be a carbonate or hydroxide based alkalinity part.”
I am fairly new to this, and have been using B-Ionic 2-part system. How might I determine how their alkalinity part is based?
I am fairly new to this, and have been using B-Ionic 2-part system. How might I determine how their alkalinity part is based?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
Thr article indicates “ so the best choices for most people will usually be a carbonate or hydroxide based alkalinity part.”
I am fairly new to this, and have been using B-Ionic 2-part system. How might I determine how their alkalinity part is based?
Normal B-ionic is carbonate based. They also sell, or used to sell, a bicarbonate version called B-ionic Bicarbonate.
I will also ask whether dosing calcium chloride in larger amounts than NaOH and salt without NaCl (these are equal) may affect the ionic balance?OK, that ratio is fine since you are using anhydrous calcium chloride.
Thanks for the excellent article @Randy Holmes-Farley, if I am understanding this, and I'm not, a two part that uses sodium hydroxide as its alkalinity component is going to offer the highest pH boost (dosing multiple times a day)? Are there any commercially available two part systems that use sodium hydroxide, or is DIY the only option for this?
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
Thanks for the excellent article @Randy Holmes-Farley, if I am understanding this, and I'm not, a two part that uses sodium hydroxide as its alkalinity component is going to offer the highest pH boost (dosing multiple times a day)? Are there any commercially available two part systems that use sodium hydroxide, or is DIY the only option for this?
I'm not sure if there are commercial systems at the moment. There are a number of stand alone hydroxide additives from several companies, but I do not recall seeing it as part of a two part.
I have used your 2 part recipes religiously on my 340 gallon mixed reef for 8 years. And so far, the corals that live do awesome. Those that do not, do not. (Elegance, acans, and plate corals all fail miserably). On the other hand SPS - Acros, chalice, Hydnophora, LPS - Hammers, frog spawn, octospawn all do amazing, softies, like yuma mushrooms do great.
That said, I make up 2 cups baked baking soda per gallon of RODI and 2 cups calcium Chloride to 1 gallon RODI. And I use my doser to dose equal amounts per day of each. Whenever I test, alk fluctuates between 9-10 and Calcium between 500-525.
I do a ratio of magnesium chloride with magnesium sulfate as my third part. This I mix the maintenance dose according to the BRS recipe for 1 gallon of water and dose at much lower volumes.
Questions
In dosing a maintenance dose (in ml per day), if I'm doing 192 ml per day of each - 8ml per hour alk and calcium), how much magnesium should be dosed? Please note, no matter what I dose, when I test, the results are at 1500-1540. Even if I don't dose for a few months. (No water changes)
Trace element dosing is confusing. (Beyond confusing) I use the Red Sea A, B, C, D trace elements and it states on the bottle to dose based on calcium. Google says: BRS recommends that you dose the trace colors at roughly 1/50 of their calcium solution. If you want to dose them once per week you're looking at 8.8ml of each a,b,c,d, or roughly 1.25ml daily. This is all based on your tank being 110 gallons.
So, based on the ratios, 8.8 ml of each for 110 gallons * 3 = 26.4 ml a week for 340 gallons. Do the trace elements impact any of the three part dosing in any way? I dose around 30 ml of each once per week. I could get more precise and do 26 ml once per week. I also dose Chaeto grow in the same ratio (for my algae turf scrubber). Which I also do 30 ml on the same day as the trace element dosing.
One any of these have negative effects on corals? There's many other factors such as phosphates that cause algae to grow faster and that I'm sure also depletes some if not all the trace elements in a matter of a day or two.
How does PH, bioload, and other parameters factor into alk consumption?
I noticed there was a threshhold. When I hit 10 ml per hour of 2 part. I had a significant jump in PH. My low went from 7.8 to 8 at night and during the day it hit 8.1. There was some type of growth spurt then slow down over about a month. I had to slow it back down from 240 ml per day to 192 ml per day. And now my ph dropped back to 7.8 at night and barely hitting 8 during the day. Alk hit 10dkh at that time as well, which my corals don't like. So, had to back off.
Coincidently my skimmer that I hadn't touched in months suddenly started producing more waste. Went through 5 gallons of skimmate in two weeks. Then it also settled back down. At the same time I doubled my fish load from 12 to 24. I don't know how alk, skimmate, and coral growth play into alk and calcium, but alk started dropping fast, so, had to boost dosing, then started rising by .3 per day. Then slowly backed off and it's now settled back to being stable around 9 ish.
I didn't change my trace element or part 3 dosing at all during this time. Trying to keep things stable while managing changes.
That said, I make up 2 cups baked baking soda per gallon of RODI and 2 cups calcium Chloride to 1 gallon RODI. And I use my doser to dose equal amounts per day of each. Whenever I test, alk fluctuates between 9-10 and Calcium between 500-525.
I do a ratio of magnesium chloride with magnesium sulfate as my third part. This I mix the maintenance dose according to the BRS recipe for 1 gallon of water and dose at much lower volumes.
Questions
In dosing a maintenance dose (in ml per day), if I'm doing 192 ml per day of each - 8ml per hour alk and calcium), how much magnesium should be dosed? Please note, no matter what I dose, when I test, the results are at 1500-1540. Even if I don't dose for a few months. (No water changes)
Trace element dosing is confusing. (Beyond confusing) I use the Red Sea A, B, C, D trace elements and it states on the bottle to dose based on calcium. Google says: BRS recommends that you dose the trace colors at roughly 1/50 of their calcium solution. If you want to dose them once per week you're looking at 8.8ml of each a,b,c,d, or roughly 1.25ml daily. This is all based on your tank being 110 gallons.
So, based on the ratios, 8.8 ml of each for 110 gallons * 3 = 26.4 ml a week for 340 gallons. Do the trace elements impact any of the three part dosing in any way? I dose around 30 ml of each once per week. I could get more precise and do 26 ml once per week. I also dose Chaeto grow in the same ratio (for my algae turf scrubber). Which I also do 30 ml on the same day as the trace element dosing.
One any of these have negative effects on corals? There's many other factors such as phosphates that cause algae to grow faster and that I'm sure also depletes some if not all the trace elements in a matter of a day or two.
How does PH, bioload, and other parameters factor into alk consumption?
I noticed there was a threshhold. When I hit 10 ml per hour of 2 part. I had a significant jump in PH. My low went from 7.8 to 8 at night and during the day it hit 8.1. There was some type of growth spurt then slow down over about a month. I had to slow it back down from 240 ml per day to 192 ml per day. And now my ph dropped back to 7.8 at night and barely hitting 8 during the day. Alk hit 10dkh at that time as well, which my corals don't like. So, had to back off.
Coincidently my skimmer that I hadn't touched in months suddenly started producing more waste. Went through 5 gallons of skimmate in two weeks. Then it also settled back down. At the same time I doubled my fish load from 12 to 24. I don't know how alk, skimmate, and coral growth play into alk and calcium, but alk started dropping fast, so, had to boost dosing, then started rising by .3 per day. Then slowly backed off and it's now settled back to being stable around 9 ish.
I didn't change my trace element or part 3 dosing at all during this time. Trying to keep things stable while managing changes.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
In dosing a maintenance dose (in ml per day), if I'm doing 192 ml per day of each - 8ml per hour alk and calcium), how much magnesium should be dosed? Please note, no matter what I dose, when I test, the results are at 1500-1540. Even if I don't dose for a few months. (No water changes)
610 mL per gallon of calcium dosed, or 16 mL of mag part per 100 mL of calcium part.
I'll have to come back to the other questions.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
Trace element dosing is confusing. (Beyond confusing) I use the Red Sea A, B, C, D trace elements and it states on the bottle to dose based on calcium. Google says: BRS recommends that you dose the trace colors at roughly 1/50 of their calcium solution. If you want to dose them once per week you're looking at 8.8ml of each a,b,c,d, or roughly 1.25ml daily. This is all based on your tank being 110 gallons.
So, based on the ratios, 8.8 ml of each for 110 gallons * 3 = 26.4 ml a week for 340 gallons. Do the trace elements impact any of the three part dosing in any way? I dose around 30 ml of each once per week. I could get more precise and do 26 ml once per week. I also dose Chaeto grow in the same ratio (for my algae turf scrubber). Which I also do 30 ml on the same day as the trace element dosing.
Trace element dosing is a complicated question, and there's no perfect answer to how to do it. The 30 mL vs 26 mL is a pure guess that some person at some company provides that knows nothing about your tank.
I discuss these sorts of issues in these two recent articles:
Randy's Elements to Dose
In a previous article I discussed my thoughts on trace elements https://www.reef2reef.com/ams/randys-thoughts-on-trace-elements.951/ This article expands on that discussion by providing specific guidance for all elements one might dose. First...
 www.reef2reef.com
www.reef2reef.com

Randy's thoughts on trace elements
Randy's thoughts on trace elements in reef aquaria.
 www.reef2reef.com
www.reef2reef.com
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,438
- Reaction score
- 63,827
How does PH, bioload, and other parameters factor into alk consumption?
Higher alk and higher pH generally lead to higher alk and calcium consumption.
Bioload per se does not impact alk consumption, just how many and what types of organisms deposit calcium carbonate.
Thank you for the references, this definitely helps me feel less dumb about the whole situation. Some people are in the camp they would never dose something they can't test for. But, this helps the arguement that no test is perfect and no method is perfect. The ocean itself doesn't give us the answers.Trace element dosing is a complicated question, and there's no perfect answer to how to do it. The 30 mL vs 26 mL is a pure guess that some person at some company provides that knows nothing about your tank.
I discuss these sorts of issues in these two recent articles:
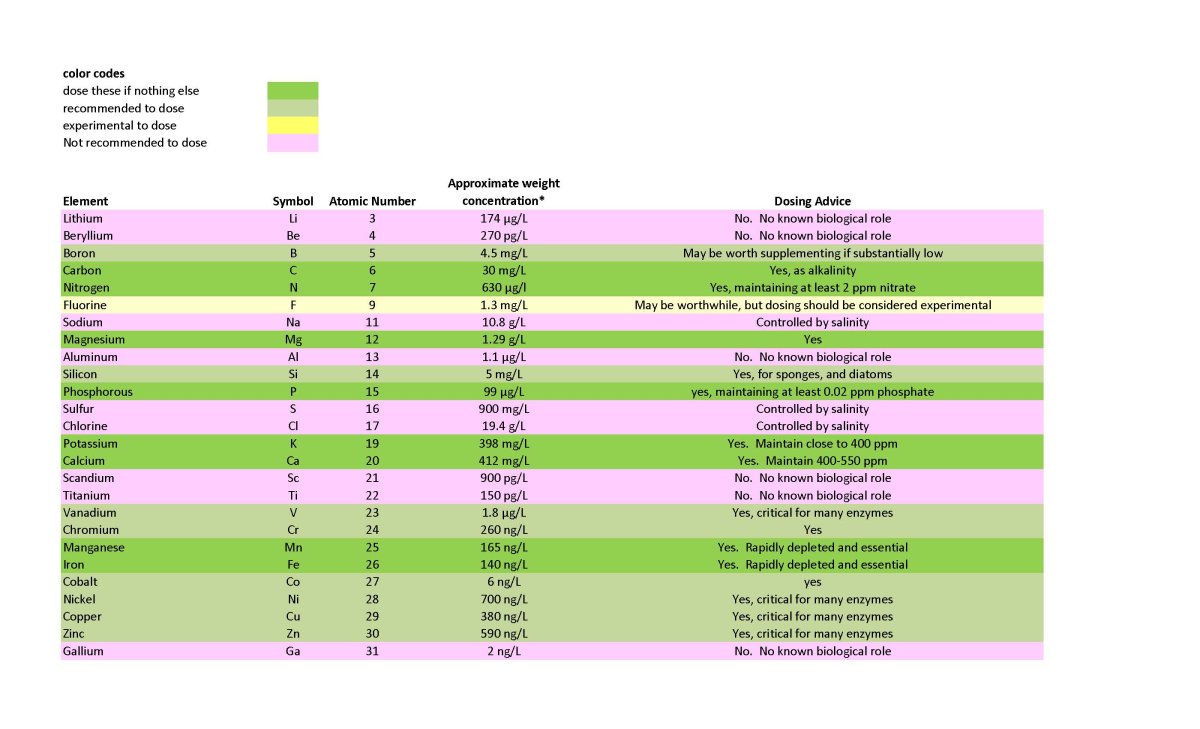
Randy's Elements to Dose
In a previous article I discussed my thoughts on trace elements https://www.reef2reef.com/ams/randys-thoughts-on-trace-elements.951/ This article expands on that discussion by providing specific guidance for all elements one might dose. First...www.reef2reef.com

Randy's thoughts on trace elements
Randy's thoughts on trace elements in reef aquaria.www.reef2reef.com
I may be spending money on trace elements that do nothing. I don't know. Or maybe they just help my algae turf scrubber grow better. (Seems like expensive hair algae if that's the case). But in the end I saw a decline in my coral health when I switched from Red Sea trace elements to only dosing all for reef. Calcium and alk remained stable, but, corals definitely were not as open, didn't seem to grow as well, and some even declined while using all for reef - got some for free and used it for around 2 months). I switched back to my old methods and within the next two months corals were way happier. I don't know if removing the trace elements they would decline. I suspect so, given that I don't do water changes. Food would be the only source of trace elements then.
I don't believe Red Sea has the perfect solution. At the same time, I don't want to be a chemist with a bunch of individual chemicals, so, I like that they've bottled major ones into 4 bottles. Are they a marketing gimmick, I caan't say. I do know my algae scrubber bleaches out and stops growing hair algae if I don't dose enough or stop dosing. Heh.
Hi Randy, i've read your article about improve alk dosing by heating up NaHCo3, in our country they provide 2 products that is NaHCO3 and Na2Co3, can i choose Na2Co3 instead of heating up NaHCO3 like you said ? And if possible, what is the dose ? Thank you !
Attachments
Thank you Andy. Excellent article, as always.
It's a pity that companies do not clearly specify what they sell in their bottles but I guess that is part of the bussines game. Unfortunately, the conclusion is that one can not make precise cause-effect correlations when a certain system works better or worse in our tank, except saying that system is better or worse... for my tank
It's a pity that companies do not clearly specify what they sell in their bottles but I guess that is part of the bussines game. Unfortunately, the conclusion is that one can not make precise cause-effect correlations when a certain system works better or worse in our tank, except saying that system is better or worse... for my tank
Similar threads
- Replies
- 8
- Views
- 136
-
- AMS: Article
- Replies
- 34
- Views
- 2,569
- Replies
- 4
- Views
- 153
- Replies
- 9
- Views
- 385













