- Joined
- May 22, 2016
- Messages
- 6,555
- Reaction score
- 10,119
(I got this concept - and the first data - from Dan_P. )
Short version:
Take Red Sea NO3 test, develop it, run it through the Hanna ULR P checker.
Calculate your NO3 as follows
(ppm NO3) = 0.00765 x (Hanna reading) + 0.117
Now for the details. First, take the tank water and precisely dilute it down to 1/10 of original with some trusted total zero NO3 source - I've use distilled water mostly. From now on this 1/10 dilution is the "sample."
I use the Red Sea NO3/NO2 kit that calls for 5ml sample. Double the sample and all reactants so I have 10ml, 10 drops A, 2 scoops B etc. During the 9 min developing time, I use another 10ml (diluted) sample water as the blank "C1" in Hanna checker. Then empty it, and pour in the developed pink red sea test into the Hanna cuvette as "C2". I time it so that Hanna checker runs "C2" right at the 9 min development time of the Red sea test - start the 3 min countdown at 6:00 into development time - easy.
Do all the normal obsessing about bubbles and smudges that we love about Hanna checkers.
Put the readout from Hanna into that formula above. This gives the sample NO3 concentration in ppm. The Hanna checker is very sensitive and will only read NO3 concentrations between ~0.1 and ~1.5 ppm. This is why the first step is to get a 1/10 dilution so a normal reef tank running anywhere between 1ppm and 15ppm will get a reading.
Data details
Below are the data sets that were generated from known mixed NO3 solutions. Blue is the first data from Dan_P using a different Red Sea NO3 kit (Nitrate pro reef) he scaled down to give 10 mL of sample. (Dan's directions at bottom of post)
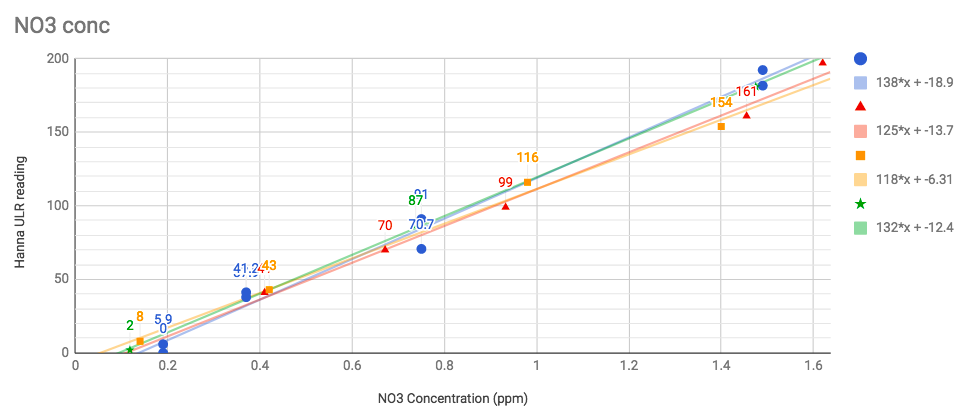
Red, Orange and Green are my sample series from different stocks I mixed. As you can see there's as much variation between my different data runs as there was between mine and Dan's data. I take that to mean it's probably not crazy to lump it all together.
So here it is - X and Y flipped now.
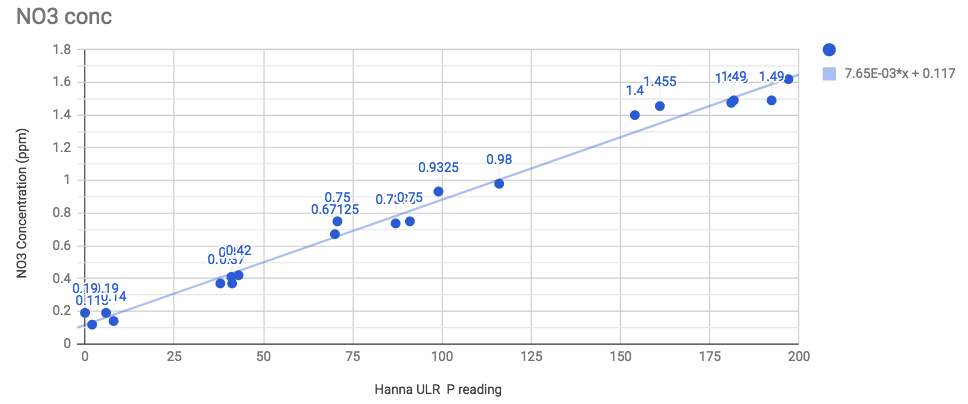
That trend line is y = 0.007653*x + 0.1169 which is where the formula came from. Note that getting the NO3 prediction from the Hanna reading (trend line) and then comparing that to the actual known concentration would give you an error in every case of 0.1ppm or less.
average error between the line and a known concentration point= 0.048ppm and 5.3% of value (for larger values).
At the extremes - 0.1ppm, you run a 50/50 risk of hanna reading "0" and at 1.6ppm, a 50/50 risk of the meter maxing a flashing "200"
Will this trend hold as tightly for other people as it did for Dan and myself? Give it a try let us know what you think! If you have access to accurate methods for mixing known concentration solutions you can help improve the fit line or find a breakdown in the method.
Dan's instructions for the Red Sea Nitrate Pro kit.
Short version:
Take Red Sea NO3 test, develop it, run it through the Hanna ULR P checker.
Calculate your NO3 as follows
(ppm NO3) = 0.00765 x (Hanna reading) + 0.117
Now for the details. First, take the tank water and precisely dilute it down to 1/10 of original with some trusted total zero NO3 source - I've use distilled water mostly. From now on this 1/10 dilution is the "sample."
I use the Red Sea NO3/NO2 kit that calls for 5ml sample. Double the sample and all reactants so I have 10ml, 10 drops A, 2 scoops B etc. During the 9 min developing time, I use another 10ml (diluted) sample water as the blank "C1" in Hanna checker. Then empty it, and pour in the developed pink red sea test into the Hanna cuvette as "C2". I time it so that Hanna checker runs "C2" right at the 9 min development time of the Red sea test - start the 3 min countdown at 6:00 into development time - easy.
Do all the normal obsessing about bubbles and smudges that we love about Hanna checkers.
Put the readout from Hanna into that formula above. This gives the sample NO3 concentration in ppm. The Hanna checker is very sensitive and will only read NO3 concentrations between ~0.1 and ~1.5 ppm. This is why the first step is to get a 1/10 dilution so a normal reef tank running anywhere between 1ppm and 15ppm will get a reading.
Data details
Below are the data sets that were generated from known mixed NO3 solutions. Blue is the first data from Dan_P using a different Red Sea NO3 kit (Nitrate pro reef) he scaled down to give 10 mL of sample. (Dan's directions at bottom of post)
Red, Orange and Green are my sample series from different stocks I mixed. As you can see there's as much variation between my different data runs as there was between mine and Dan's data. I take that to mean it's probably not crazy to lump it all together.
So here it is - X and Y flipped now.
That trend line is y = 0.007653*x + 0.1169 which is where the formula came from. Note that getting the NO3 prediction from the Hanna reading (trend line) and then comparing that to the actual known concentration would give you an error in every case of 0.1ppm or less.
average error between the line and a known concentration point= 0.048ppm and 5.3% of value (for larger values).
At the extremes - 0.1ppm, you run a 50/50 risk of hanna reading "0" and at 1.6ppm, a 50/50 risk of the meter maxing a flashing "200"
Will this trend hold as tightly for other people as it did for Dan and myself? Give it a try let us know what you think! If you have access to accurate methods for mixing known concentration solutions you can help improve the fit line or find a breakdown in the method.
Dan's instructions for the Red Sea Nitrate Pro kit.
1-Add 0.16 mL liquid Reagent 1 to the 10 mL sample in the Red Sea test vial.
2-Add 0.1 mL powder Reagent 3 to clean, empty Hanna vial. I use the 0.05 mL red scoop from a Salifert test kit
3-Add 0.1 mL powder Reagent 2 to the test sample and shake vigorously for 1 minute only. It seems that the intermediate formed in this step is not stable.
4-Using a 0.2 or 0.4 micron syringe filter that is compatible with acid, not nylon, (I bought mine through Amazon), filter the sample into the Hanna vial containing Reagent 3 and mix. Hold for 9 minutes. Don’t skip the filtration. Beside removing particulate matter, the filter removes carryover of solids into the second reactions which often diminishes the color intensity. This might not be visible to the naked eye but is definitely measurable.
5-Zero the Hanna Checker with an RO/DI blank (C1). Be sure to confirm before the test that the blank vial and the test vial are matched. Most Hanna vials are. Then take the reading of the developed test sample (C2).



















