@taricha I reran the test with a 1/10 dilution with RODI as directed. Hanna checker display 47. Throwing that into the equation (47*0.00765)+0.1169 returns 0.47645. In your first post, you said this "gives the sample NO3 concentration in ppm". Is this the concentration of the sample from my tank or the concentration of the 1/10 diluted sample so I would need to multiply by 10 to get the actual tank water concentration? Thanks!
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Accurate NO3 Test with Hanna ULR P Checker
- Thread starter taricha
- Start date
- Tagged users None
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
Hanna checker display 47. Throwing that into the equation (47*0.00765)+0.1169 returns 0.47645. In your first post, you said this "gives the sample NO3 concentration in ppm". Is this the concentration of the sample from my tank or the concentration of the 1/10 diluted sample so I would need to multiply by 10 to get the actual tank water concentration?
yeah, that formula gives you the NO3 concentration of the 1/10 dilution.
...so multiply by 10 to get your tank water ~ 4.8ppmFrom now on this 1/10 dilution is the "sample."
Dang, I was really hoping it was the former. The sample I had on Tuesday undiluted was almost clear so I was nearly certain that .48 ppm was correct. I'll run the test again tomorrow to see how close it is to the 4.8.yeah, that formula gives you the NO3 concentration of the 1/10 dilution.
...so multiply by 10 to get your tank water ~ 4.8ppm
Are others surprised by a number quite a bit higher than they thought it would be when using the color charts or are most people just getting a more accurate result?
@taricha Are the droppers for the Reagent A measuring .05 ml per drop? If so, I'd like to use a 1 ml syringe to measure the .5 ml of reagent needed. Then, are the weights known for the needed amounts of reagent B and C? I'm thinking a scale would be the best way to get accurate measurements of the powder reagents. In your testing of this method, did you ever have some sloppier tests that resulted in readings off by almost ten fold? If the color and the Hanna result seem off, the error is more likely being introduced in something on the Hanna side like getting the RODI C1 measurement, any particulate in the sample, smudges on the cuvette, etc. I'm using the same cuvette for C1 and C2 by dumping it out using a syringe to transfer the sample from the Red Sea cuvette to the Hanna cuvette. Which when I think of it now, I could have added more tank water to the diluted sample if some was left in the syringe after filling getting the 1ml of tank water.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
In your testing of this method, did you ever have some sloppier tests that resulted in readings off by almost ten fold?
Not seen errors like that. Errors were +-0.1 I the sample which multiply to +-1.0ppm in the tank water.
A couple of comments.
I use a 5ml syringe (5+4) to get 9.0ml distilled water. Then a separate 1.00ml syringe to get 1.00ml of tank water.
10 drops of A, I've never measured - but the fact it's 10 drops ought to reduce randomness. For the scoops - scrape the scoop level with card etc.
For the particulates in pink solution, shake several times during the 9 minute color development time. Then let sit for a min or so and pour most but not every bit into cuvette. This keeps particulates out of mine.
You are right to use same cuvette for c1,c2 but just pour in the developed red sea into the Hanna cuvette.
"C1" is not RoDi, its the same 1/10 dilution of tank water.
Obsess about bubbles and smudges.
(facepalm) Why did I think C1 was RODI? Ok, so first change that. I was using a 5ml syringe to get my RODI, I'll keep doing that but I'll use a 1ml syringe for tank water rather than the same 5ml syringe. I used that same syringe to move the water from RS cuvette to Hanna cuvette, likely pulling particulate from the bottom. I'll pull from just below the surface and not grab all of it. Excited to try again today! Thanks for the help, too.Not seen errors like that. Errors were +-0.1 I the sample which multiply to +-1.0ppm in the tank water.
A couple of comments.
I use a 5ml syringe (5+4) to get 9.0ml distilled water. Then a separate 1.00ml syringe to get 1.00ml of tank water.
10 drops of A, I've never measured - but the fact it's 10 drops ought to reduce randomness. For the scoops - scrape the scoop level with card etc.
For the particulates in pink solution, shake several times during the 9 minute color development time. Then let sit for a min or so and pour most but not every bit into cuvette. This keeps particulates out of mine.
You are right to use same cuvette for c1,c2 but just pour in the developed red sea into the Hanna cuvette.
"C1" is not RoDi, its the same 1/10 dilution of tank water.
Obsess about bubbles and smudges.
Ran the test again this evening. 1ml tank water, 9ml RODI for my C1. Then 1ml tank water, 9ml RODI to add reagent to. 10 drops of A, 2 scoops of B, 2 scoops of C. Shook for the time indicated on the instruction card. Started my 9-minute timer. At 5 minutes remaining, I started the C1 sample on the Hanna after cleaning the cuvette and clearing microbubbles. Between 5 minutes remaining and 3 minutes remaining, I emptied the cuvette of C1 and added C2. I didn't pull off the bottom, no particulates, microbubbles, or smudges. Started the 3 minute Hanna countdown at 3 minutes remaining of development time. This time I got 127 on the Hanna so a NO3 reading of 10.656 ppm.
Now that I was reading a higher NO3 level than I expected, I wanted to see what kind of a reading I would get if I ran the Red Sea kit just as directed. The result looks somewhere between 2 and 5ppm to me. How is anyone supposed to read this as 10ppm?!
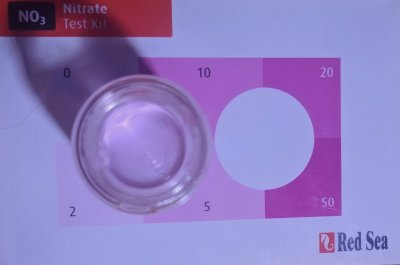
Now that I was reading a higher NO3 level than I expected, I wanted to see what kind of a reading I would get if I ran the Red Sea kit just as directed. The result looks somewhere between 2 and 5ppm to me. How is anyone supposed to read this as 10ppm?!

I wish I could send you a sample of my tank water! I forgot to add that I used a recipe card to level off the scoops too. My Hanna ULR phosphorous checker is probably 5 or 6 months old. The phosphate levels I measure with it have been in line with Triton ICP so I don't think it's the checker. The 4.8 ppm I got when I used RODI as the C1 is more in line with what the Red Sea kit shows. It's the Red Sea Marine Care Test Kit I'm using.Looks like enormous disagreements. Gonna have to think about this one.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
I'll go to PM and we'll see if we can figure out what's messed up.I wish I could send you a sample of my tank water! I forgot to add that I used a recipe card to level off the scoops too. My Hanna ULR phosphorous checker is probably 5 or 6 months old. The phosphate levels I measure with it have been in line with Triton ICP so I don't think it's the checker. The 4.8 ppm I got when I used RODI as the C1 is more in line with what the Red Sea kit shows. It's the Red Sea Marine Care Test Kit I'm using.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
for others following along - after PM, looks like pluikens solved it. When doing the 1/10 dilution step, it's critical to get the amounts right. Inaccuracy in the 1 part in 10 will make for especially large error.
@pluikens remeasurement "...And the Hanna showed 22 for a ppm of 2.85 ppm. That definitely matches up with the Red Sea card I posted yesterday."
@pluikens remeasurement "...And the Hanna showed 22 for a ppm of 2.85 ppm. That definitely matches up with the Red Sea card I posted yesterday."
And @taricha is awesome for all of the help!for others following along - after PM, looks like it's solved. When doing the 1/10 dilution step, it's critical to get the amounts right. Inaccuracy in the 1 part in 10 will make for especially large error.
@pluikens remeasurement "...And the Hanna showed 22 for a ppm of 2.85 ppm. That definitely matches up with the Red Sea card I posted yesterday."
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
Well, I'm afraid I must retract my previous statement about the Red Sea NO2/NO3 test and NO3 Pro tests giving the same results; they most definitely do not. I only did one comparison the other day, and must have made some error that had them come out the same. Repeating the comparison several times today, it's clear now to me that using the Nitrate Pro reagents to perform the test as described here gives much lower results than with the NO2/NO3 reagents. I also find that the results are less consistent when the test is performed with the Pro reagents. This may in part be because the Pro reagents generate a larger amount of microbubbles, and there is also more dark particulate material in the vial at the end of the reaction. Also, looking at the chemicals themselves, the C reagent has a different chemical listed on its label, and the B reagent may have different proportions of the same components. So, at this point, the NO3 Pro reagents do not appear to work properly with this technique. I will defer to taricha for confirmation.
finally got around to trying the Nitrate Pro Reef test to see if there were differences in the reagents themselves.
A world of difference - using the Pro reagents as though they were the no3/no2 marine reagents gives complete nonsense.
On the right, the no3/no2 marine reagents used in this method - pink right in the heart of the hanna meter's range, and clean with no particulates or bubbles (after several shakes during 9 minutes). Left is using the Pro reagents - lots of black/gray particles, tons of bubbles, and so much interference that no pink was formed at all.
(This sample was ~11ppm tank water, diluted 1/10 with distilled water before testing)
These are very different reagents and if you wish to use the Pro, then check @Rick Mathew work here
https://www.reef2reef.com/threads/u...-hi-736-to-test-for-low-levels-of-no3.350062/
finally got around to trying the Nitrate Pro Reef test to see if there were differences in the reagents themselves.
A world of difference - using the Pro reagents as though they were the no3/no2 marine reagents gives complete nonsense.

On the right, the no3/no2 marine reagents used in this method - pink right in the heart of the hanna meter's range, and clean with no particulates or bubbles (after several shakes during 9 minutes). Left is using the Pro reagents - lots of black/gray particles, tons of bubbles, and so much interference that no pink was formed at all.
(This sample was ~11ppm tank water, diluted 1/10 with distilled water before testing)
These are very different reagents and if you wish to use the Pro, then check @Rick Mathew work here
https://www.reef2reef.com/threads/u...-hi-736-to-test-for-low-levels-of-no3.350062/
Good Information to have...thanks for your work...Like I said I have never used the NO2/NO3 test kit...I have done all of my work with the Pro Kit....
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
Ok, two issues I wanted to look at more closely: repeatability, and variation with salinity. Tried to do 2 birds with one stone.
Took a sample of tank water (~6ppm NO3 - 34.5ppt), did dilutions of 1 ml tank water with...
9 ml of either distilled, or zero NO3 SW @36ppt, or 2ml/7ml combinations of the two. Duplicates of everything to look at repeatability.
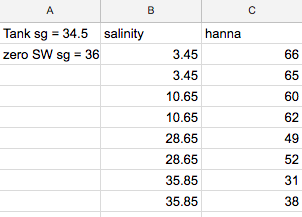
Graph to see if it's linear....
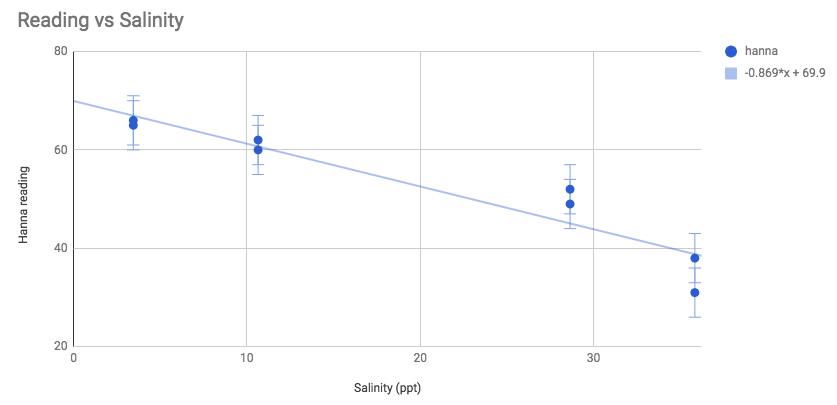
maybe linear . Maybe a curve fits better. On the repeatability/precision - it compares favorably with hanna's stated +-5 uncertainty.
Let's see if we can put it together with Rick's data from here...
Scaled both data sets so that the hanna reading at 35ppt = 100% and combined them.
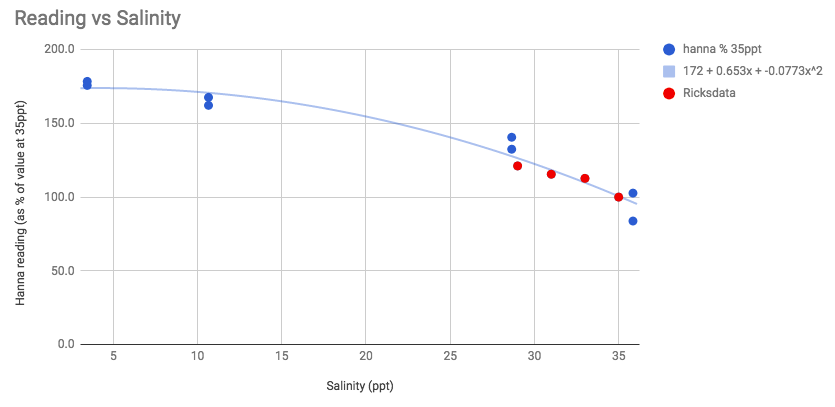
Maybe I'm overfitting - and it's just linear, but that curve looks nice.
If the curve isn't just my imagination, this suggests that +-1ppt of salinity is a bigger deal at 35ppt than it is at very low salinities.
Either way, it looks like readings taken at 1/10 of full salinity read as ~177% of those taken at 35ppt.
Took a sample of tank water (~6ppm NO3 - 34.5ppt), did dilutions of 1 ml tank water with...
9 ml of either distilled, or zero NO3 SW @36ppt, or 2ml/7ml combinations of the two. Duplicates of everything to look at repeatability.
Graph to see if it's linear....
maybe linear . Maybe a curve fits better. On the repeatability/precision - it compares favorably with hanna's stated +-5 uncertainty.
Let's see if we can put it together with Rick's data from here...
Here is the actual data showing the effects of salinity on the measurement using this test method...
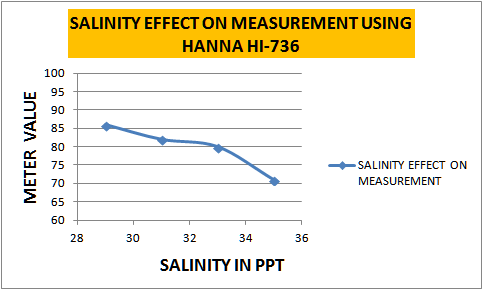
Scaled both data sets so that the hanna reading at 35ppt = 100% and combined them.
Maybe I'm overfitting - and it's just linear, but that curve looks nice.
If the curve isn't just my imagination, this suggests that +-1ppt of salinity is a bigger deal at 35ppt than it is at very low salinities.
Either way, it looks like readings taken at 1/10 of full salinity read as ~177% of those taken at 35ppt.
- Joined
- May 22, 2016
- Messages
- 6,547
- Reaction score
- 10,108
Bottom line for the method in this thread: It presumes 1/10 diluted with distilled water. If you test straight 35ppt tank water, then run it through the equation in the first post, you will need to multiply by ~1.77 to get your final NO3 ppm value.
in equation form...
(ppm NO3) = 1.77 x [0.00765 x (Hanna reading) + 0.117]
If testing straight 35ppt saltwater.
oh, and for the truly nerdy - the difference in the slope in the equation in rick's thread
and the slope in the equation in this thread is a factor of 1.694. Which is to say that the 1.77 salinity correction factor between 35ppt and 1/10 salinity accounts for 95.7% of the difference between these two threads.
in equation form...
(ppm NO3) = 1.77 x [0.00765 x (Hanna reading) + 0.117]
If testing straight 35ppt saltwater.
oh, and for the truly nerdy - the difference in the slope in the equation in rick's thread
and the slope in the equation in this thread is a factor of 1.694. Which is to say that the 1.77 salinity correction factor between 35ppt and 1/10 salinity accounts for 95.7% of the difference between these two threads.
Ok, two issues I wanted to look at more closely: repeatability, and variation with salinity. Tried to do 2 birds with one stone.
Took a sample of tank water (~6ppm NO3 - 34.5ppt), did dilutions of 1 ml tank water with...
9 ml of either distilled, or zero NO3 SW @36ppt, or 2ml/7ml combinations of the two. Duplicates of everything to look at repeatability.

Graph to see if it's linear....
maybe linear . Maybe a curve fits better. On the repeatability/precision - it compares favorably with hanna's stated +-5 uncertainty.
Let's see if we can put it together with Rick's data from here...
Scaled both data sets so that the hanna reading at 35ppt = 100% and combined them.

Maybe I'm overfitting - and it's just linear, but that curve looks nice.
If the curve isn't just my imagination, this suggests that +-1ppt of salinity is a bigger deal at 35ppt than it is at very low salinities.
Either way, it looks like readings taken at 1/10 of full salinity read as ~177% of those taken at 35ppt.
Actually I don't think you are over fitting the regression...In my work with changing to the Nitrite Meter...(which I have yest to complete
Nice Work!!
Hey I just purchased a Hanna phosphate checker. But mine isn't the Ultra low version, just the regular. Will using the Red Sea nitrate reagent work with this checker for checking nitrate? Or is the Ultra low version required?
Just for consistency (albeit may not matter much)- why not make your C1 the same composition as the test vial? If you are testing 1/10th sea water make your C1 vial the same?
Similar threads
- Replies
- 12
- Views
- 535
- Replies
- 37
- Views
- 656
- Replies
- 4
- Views
- 250
- Replies
- 18
- Views
- 447
- Replies
- 5
- Views
- 190
New Posts
-
What is wrong with my tank? I'm open to any suggestion.
- Latest: MontyNowAReefer
-
Florida Live Goods Corals available: torches, Gonis, and anemones
- Latest: smitten with ocean life
-
-


















