Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Alk Swing @ Night
- Thread starter Budman's Corals
- Start date
- Tagged users None
- Joined
- Jul 19, 2016
- Messages
- 96
- Reaction score
- 45

On the topic of Alk monitor, a couple of guys in Asia developed a piece of equipment that claims to do it:
https://www.facebook.com/groups/1768033033431949/
The technique doesn't look like rocket science and I hope a more affordable and smaller versions come to market, soon.
You will see the production unit in Next month( late Aug.).
You can contact them and leave message on FB, someone will contact you.
Ill buy one! Alk is imo the most important and annoying parameter to monitor. Any updates?
Yes. I've been able to use the fact that I have alkalinity as an input signal to my Apex to control my dosing, and by doing so, I've been able to stabilize that diurnal alk swing. I'll be demonstrating a prototype at MACNA. FWIW, my device has a resolution of +/- 0.01 dKH, and a precision of +/- 0.03 dKH. Compare that to the specifications of any other device you might find out there.
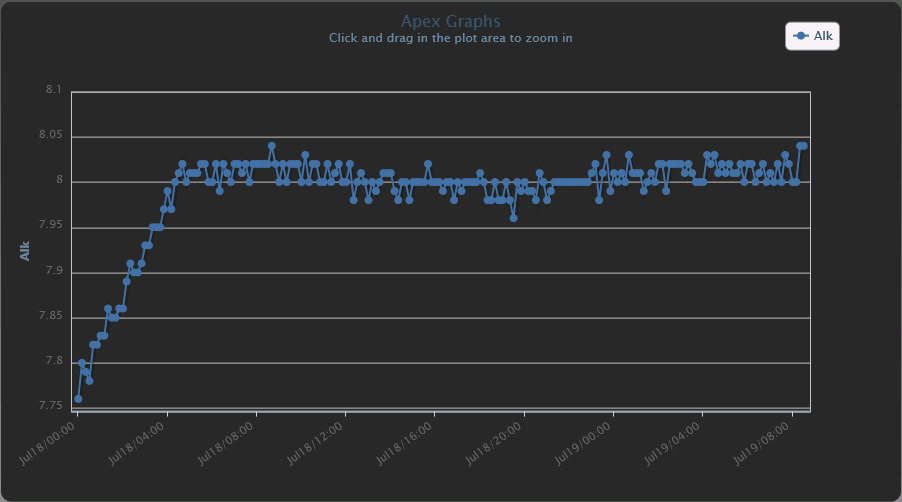
That is totally awesome. How long did it take you to set this up when you started working on it?
I've been working to find some way to accomplish this for a few years now. I finally realized fundamental success around the end of April. I've been refining it ever since.
- Joined
- Jun 1, 2017
- Messages
- 77
- Reaction score
- 51
That was my thinking, Randy. And I suspect that my tank is probably typical in that regard. However, reading through the article I linked above, "Results of this investigation demonstrate the difficulty that corals encounter in shedding waste protons generated during calcification. High rates of H+flux continued for several hours following peak Gnet" and, "...corals can cope more effectively with the problem of DIC supply compared to the problem of eliminating H+." The conclusion of the authors in this study is that pH is a bigger issue for the corals than alkalinity. Food for thought.
Sorry to resurrect an old thread, but is eliminating H+ harder at lower pH? I would guess so, since the concentration is higher, but I’m no chemist
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,930
- Reaction score
- 64,367
Sorry to resurrect an old thread, but is eliminating H+ harder at lower pH? I would guess so, since the concentration is higher, but I’m no chemist
Yes, although it might be a complicated question if you look into it at a molecular level.
The pH is always lower in the bulk aquarium water than in the region where precipitation of calcium carbonate is taking place on the surface of the coral skeleton (called the ECF, the extracytoplasmic calcifying fluid), where corals raise the pH (lower H+) to accelerate the precipitation. The lower the bulk water pH (meaning more H+), the bigger uphill chemical gradient corals have to create by pumping H+ out of the ECF and into the bulk water.
The "complications" to the "yes" answer are these...
warning, nerdy stuff ahead
1. If a protein transporter in a cell membrane is pumping H+ from a high pH environment to a low pH environment, that takes energy, and it takes more energy the bigger the H+ difference is. But the transporter may use a fixed high amount of energy to do that transport, such as using up one ATP molecule (a chemical energy storage molecule) per H+ pumped out, regardless of the size of the gradient. An analogy is firing a gun at a target a long way away. Does it take more energy to hit the target when firing into the wind? Maybe. Or maybe the gunpowder in the fixed bullets you are using is already adequate for the wind speed you are encountering.
2. I'm not certain what path the H+ takes to get from the ECF to the bulk water. If it passes through the inside of a cell (where the pH is likely even lower than in the bulk water, say, around 7), then the last step where the H+ gets into the bulk water may be to just "let it out" of the cell and into the bulk water (which might not take any energy since it is a downhill chemical gradient from pH 7 to pH 8.2). In this scenario, it takes a lot of energy to get the H+ from the ECF to the inside of the cell, and then perhaps none to the outside of the cell. Hence the external pH may not control the energy requirements of pumping h+ out of the ECF if it goes this way.
This article is old and not updated in a long time, but it describes these mechanisms as they ere known in 2004:
Aquarium Chemistry: The Chemical and Biochemical Mechanisms of Calcification ? Advanced Aquarist | Aquarist Magazine and Blog
http://www.advancedaquarist.com/2002/4/chemistry
- Joined
- Jun 1, 2017
- Messages
- 77
- Reaction score
- 51
Thanks for the explanation @Randy Holmes-Farley.
Do you or @JimWelsh know anything about the Protein Flux Hypothesis? I read about it in the paper that Jim referenced earlier, but I was only able to find a single reply to it in the literature. Perhaps it's pointless to chase, since it seems that alkalinity plays a much more important role in calcification rates than pH.
Do you or @JimWelsh know anything about the Protein Flux Hypothesis? I read about it in the paper that Jim referenced earlier, but I was only able to find a single reply to it in the literature. Perhaps it's pointless to chase, since it seems that alkalinity plays a much more important role in calcification rates than pH.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 67,930
- Reaction score
- 64,367
Thanks for the explanation @Randy Holmes-Farley.
Do you or @JimWelsh know anything about the Protein Flux Hypothesis? I read about it in the paper that Jim referenced earlier, but I was only able to find a single reply to it in the literature. Perhaps it's pointless to chase, since it seems that alkalinity plays a much more important role in calcification rates than pH.
These are two of the papers in question, the first by a researcher that came up with the hypothesis using another groups data, and the group that had the data disagreeing with the hypotheses
Response to coral reef calcification: carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3712411/
Coral reef calcification: carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification.https://www.ncbi.nlm.nih.gov/pubmed/23760863/
In essence, I think the debate is largely about what controls calcification rates, pH, bicarbonate, carbonate, or some mix of these. It's a difficult question to technically answer. Even in an isolated reef tank with a single coral, to exactly control these things is tricky, and to do so on an actual reef is very hard. That said, knowing the answer would be nice. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3712411/
Coral reef calcification: carbonate, bicarbonate and proton flux under conditions of increasing ocean acidification.https://www.ncbi.nlm.nih.gov/pubmed/23760863/
FWIW, the answer might vary with species.
Similar threads
- Replies
- 4
- Views
- 185
- Replies
- 34
- Views
- 1,779
- Replies
- 15
- Views
- 238
- Replies
- 7
- Views
- 252














