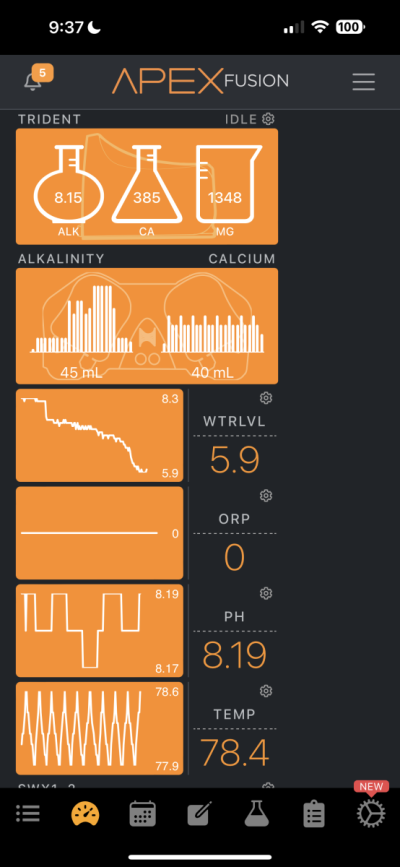
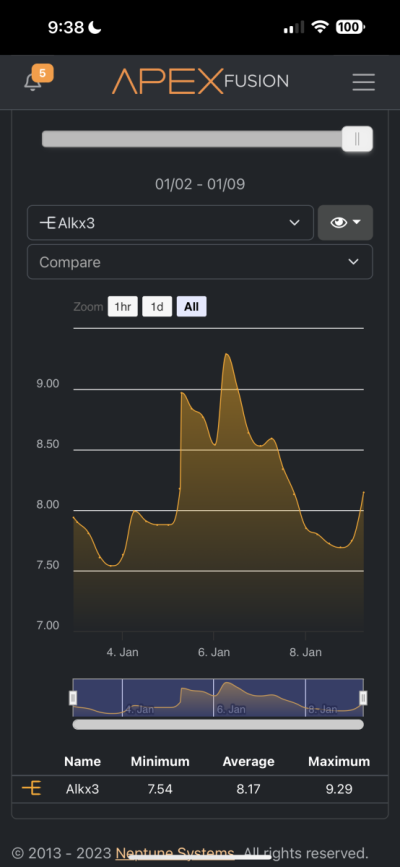
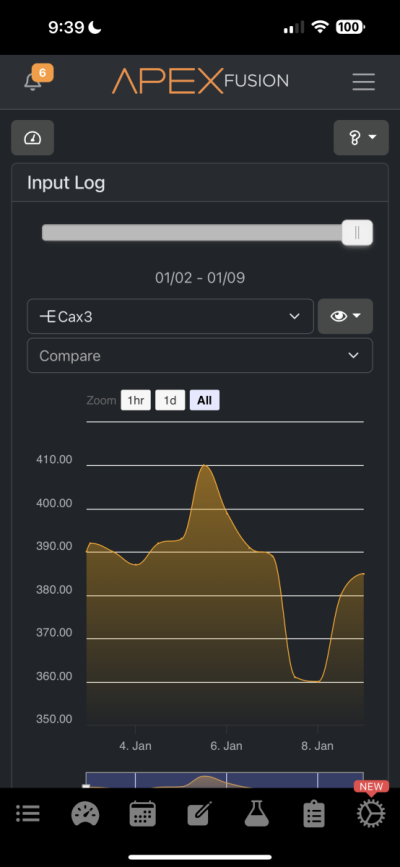
Hi all. Recently I got my hands on a Trident and it has confirmed that something is sucking the life out of my alkalinity and calcium. Before receiving the trident through my normal tests I determined that my alkalinity and calcium was being used up by something of which I have no idea. I have one single small head of GSP and my alkalinity drops by almost 1 per day without dosing and my calcium also drops like a rock. Because of this I dose about 40ml of B Ionic per day and that seems to maintain all of my levels including my pH. I do have a pretty bad CO2 issue in the room the tank is in which I resolve with a CO2 scrubber but this is the only thing I can suspect that's bringing things down. I'll attach pics of my Apex readings and just ignore the ORP reading as I think my probe went caput on me a few days ago. The small dip from the 7th-8th is because the dos pump ran dry but then I fixed it.