- Joined
- Nov 24, 2015
- Messages
- 78
- Reaction score
- 66
Thanks. There is certainly no practical use for of 4 significant figures of precision on the alk. But if the measurement is as precise as it seems to be, I don't like the idea of introducing unnecessary rounding errors when doing the conversions.
Thanks for pointing out that the and ppm conversion should take density into account. My controller is already doing a density calculation from conductivity and temperature sensors using the GSW toolbox to do the math, so no reason not to feed the density into the conversion equation.
Should the dKH conversion factor also include density?
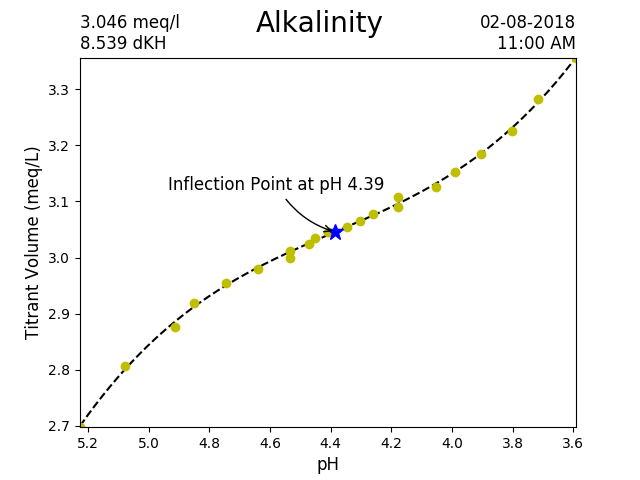
I have noticed the inflection point tends to slowly rise over the course of the day and then slowly fall back down during the night. The daily swing in the inflection point is about 0.05 pH.
Here is my most recent test (using the extra digits in the conversion, but not yet taking density into account).
Thanks for pointing out that the and ppm conversion should take density into account. My controller is already doing a density calculation from conductivity and temperature sensors using the GSW toolbox to do the math, so no reason not to feed the density into the conversion equation.
Should the dKH conversion factor also include density?
I have noticed the inflection point tends to slowly rise over the course of the day and then slowly fall back down during the night. The daily swing in the inflection point is about 0.05 pH.
Here is my most recent test (using the extra digits in the conversion, but not yet taking density into account).
Code:
Starting prime and drain.
Done
It took 2.23 minutes to prime and drain
Starting rinse.
Done
It took 3.20 minutes to rinse
Preparing sample.
Adding 17.00 ml sample and 0.4586 ml 0.1N HCl
Done
It took 2.77 minutes to prepare sample
Starting Titration.
Titrating to pH of 3.60
meq/l pH
2.6977 5.22667820069
2.8065 5.07989619377
2.8767 4.91214532872
2.9188 4.84923875433
2.9539 4.74439446367
2.9797 4.63955017301
2.9984 4.53470588235
3.0113 4.53470588235
3.0241 4.47179930796
3.0347 4.45083044983
3.0452 4.40889273356
3.0546 4.34598615917
3.0651 4.30404844291
3.0768 4.26211072664
3.0908 4.17823529412
3.1084 4.17823529412
3.1259 4.05242214533
3.1528 3.98951557093
3.1844 3.90564013841
3.2254 3.80079584775
3.2827 3.71692041522
3.3564 3.59110726644
Done
It took 14.17 minutes to titrate.
The alkalinity is 3.046 meq/L
8.539 dKH
152.4 ppm
Last edited:














