Thinking back at it, there no ph boost from all for reef. Is there a reason to dose while nights out? I see the benefit to dose a couple hours before lights on as it takes about an hour or two for Alk to rise.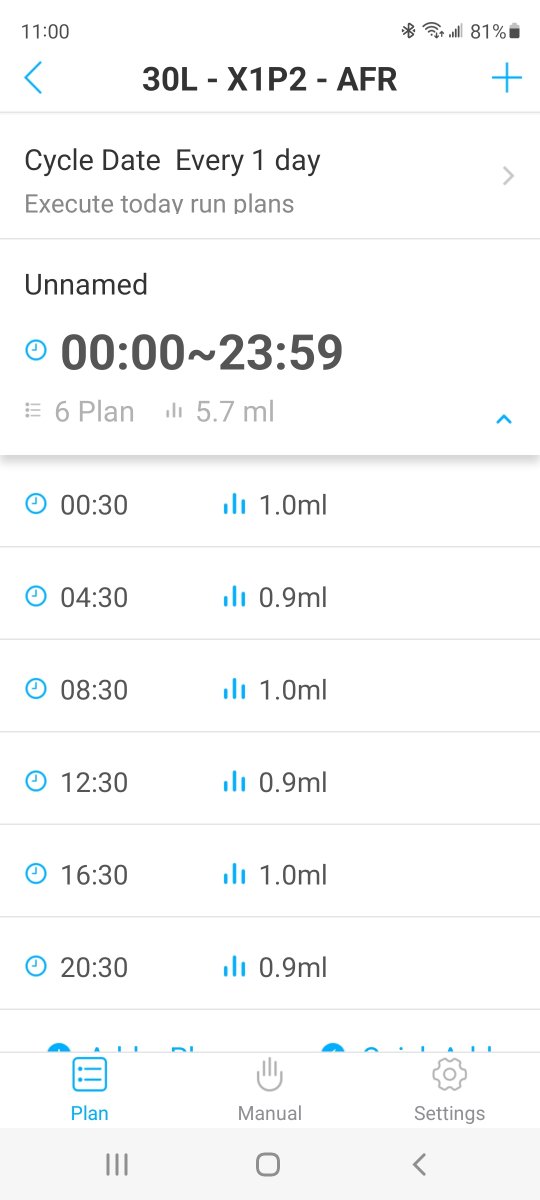
5.4ml (6x .9ml) wasn't quite enough, but 6ml (6x 1ml) was too much.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
All for Reef frustration—Help?
- Thread starter Lyss
- Start date
- Tagged users None
Thinking back at it, there no ph boost from all for reef. Is there a reason to dose while nights out? I see the benefit to dose a couple hours before lights on as it takes about an hour or two for Alk to rise.
The way I understand it, bacteria in the tank converts the formate into alkalinity. Bacteria don't sleep, so I figured the best way to keep 24 hour stability was to dose all day/night. No other reason, and my understanding of how it works might be wrong, but I've tested several times a day, before lights on, in the middle of the day and after lights out, and my tests are always very close. So I just roll with it.
So far I have been unimpressed by any of the all in ones. Seems I get an imbalance with something pretty quickly and have to dose something individually. So sorta gave up on them and just have separate components
Thanks for the insight. Ill try multiple doses and see what happens.I'm not Hans, but I can relate my own personal experience.
I dialed in my dose using the once a day, manual method. Once I put it on a pump and split it into 6 (every 4 hours) doses, I did have to bump up the total amount a bit to hit the ALK number I was shooting for, but ALK is also more stable through the day (when testing morning and evening).
Either way works, but the multiple, smaller doses gives more stability throughout the day.
I have been dosing 45 minutes before lights (I don't recall where I was told to do that). It has been 2 years without any fluctuations to speak of.
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
I think, the question has been perfectly answered by Hooz. I have nothing to add.Hans, reading along. Just starting to dose all-for-reef. I was wondering what the most effect dosing schedule would be.
This is unfortunately what happens sometimes when you try to use one product instead of individually addressing each element.I’m getting very frustrated with AFR, to the point where I’m not sure I see a benefit to using this product.
Im trying to set up my dosing on a pump, but am still hand dosing b/c I can’t get the AFR to work for me where I don’t need to supplement Calcium and Magnesium.
I dose 1ml AFR in my 20g daily. After 5 days since last testing, my Alk has risen by .3, Cal has dropped by 10, and Mag has dropped by 30. This is just not sustainable for me b/c I need to buy AFR plus Cal and Mag alone to supplement. So then what is the point of the AFR? I do have calcifying macroalgae in my tank in addition to corals that uses up a lot of calcium and grows like a weed.
Aside from pointing at possible testing errors, which could alway be but this type of result is consistent for me so hence my frustration, I’m wondering if anyone else using this product has some thoughts or advice to share. How did you get it dialed in so you can truly just be dosing AFR?
I’d suggest ESV if you want to change to something similar. It has a proven track record. It is one of the only true 2-parts on the market and the trace’s in ESV are on the lower side which is a good thing. It allows you to benefit by having some of these traces available, but keeps you from nuking your reef. The magnesium is in the calcium component and I’ve found it to be slightly under dosed in a moderate to high demand tank, but they sell a magnesium only component with a higher concentration should you need to supplement more.
You sound like a rep for ESV haha.This is unfortunately what happens sometimes when you try to use one product instead of individually addressing each element.
I’d suggest ESV if you want to change to something similar. It has a proven track record. It is one of the only true 2-parts on the market and the trace’s in ESV are on the lower side which is a good thing. It allows you to benefit by having some of these traces available, but keeps you from nuking your reef. The magnesium is in the calcium component and I’ve found it to be slightly under dosed in a moderate to high demand tank, but they sell a magnesium only component with a higher concentration should you need to supplement more.
So my Q wasn’t so much asking about replacing w/another product as it was help w/figuring out what the heck is going on. I found the below quote earlier today while googling — from Randy RE another user w/high calcium demands relative to alk — and think it answers my Q. I am doing both. Not a product issue per se, just my tank’s needs; but it means I may want to remove that halimeda for starters, and if that fails go to dosing individually instead of the all-in-one:
“If you are actively using organic carbon dosing or growing macroalgae to reduce nitrate levels that are high, that can also raise alk and not calcium.”
No, not an ESV rep. Lol. I’m just a reefer.You sound like a rep for ESV haha.
So my Q wasn’t so much asking about replacing w/another product as it was help w/figuring out what the heck is going on. I found the below quote earlier today while googling — from Randy RE another user w/high calcium demands relative to alk — and think it answers my Q. I am doing both. Not a product issue per se, just my tank’s needs; but it means I may want to remove that halimeda for starters, and if that fails go to dosing individually instead of the all-in-one:
“If you are actively using organic carbon dosing or growing macroalgae to reduce nitrate levels that are high, that can also raise alk and not calcium.”
I think dosing the elements individually is always easier. This way you can target the specific numbers you want for each element. Doesn’t have to be ESV. I just threw that out there since it’s similar to AFR with a much better track record. It’s been around since 1995 and most people that have used it love it.
Any two part will work and you could still dose trace’s on the side.
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
I disagree with this statement in this general way. Most trace metals cannot be found by ICP-OES in "normal" concentrations. I suggest to pick out one "lead" element like for example nickel, bring it to low detectable concentrations like 2 or 3 ppb, and conclude that all other trace metals dosed with this product are also in appropriate concentrations for corals. This is what I expect from a good trace element mix.I think dosing the elements individually is always easier.
In case of All-For-Reef calcium and alkalinity concentrations are the "lead parameters".
Like stated above, I think imbalances in the calcium alkalinity ratio may reflect the ratio in the salt used. If one parameter in the salt is higher or lower than in the tank, the complete system of interrelated parameters may shift.
For example, if alkalinity is lower in the tank than in the salt mix, also calcium may be lower in the tank than in the salt mix, and may finally be lower in concentration than desired.
Yes, I think what is happening for me is that my carbon dosing is working (it is, my nitrates have dropped over time from 50 to 10ppm), and in addition to the halimeda I have ulva and red macroalgae that grows on the rocks.I disagree with this statement in this general way. Most trace metals cannot be found by ICP-OES in "normal" concentrations. I suggest to pick out one "lead" element like for example nickel, bring it to low detectable concentrations like 2 or 3 ppb, and conclude that all other trace metals dosed with this product are also in appropriate concentrations for corals. This is what I expect from a good trace element mix.
In case of All-For-Reef calcium and alkalinity concentrations are the "lead parameters".
Like stated above, I think imbalances in the calcium alkalinity ratio may reflect the ratio in the salt used. If one parameter in the salt is higher or lower than in the tank, the complete system of interrelated parameters may shift.
For example, if alkalinity is lower in the tank than in the salt mix, also calcium may be lower in the tank than in the salt mix, and may finally be lower in concentration than desired.
I was thinking, the only thing it could be is a testing error or I’m adding additional alk somehow and not realizing it. Well there it is — the additional alk is coming from carbon dosing and macros using nitrate. It’s not a huge spike, but has been a small increase that I continue to see add up over time, so this makes sense to me.
The halimeda being added just put additional strain on the calcium needs of the tank. I’m going to remove a bunch of that and see if it helps enough to resolve the issue.
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,057
- Reaction score
- 64,480
Yes, I think what is happening for me is that my carbon dosing is working (it is, my nitrates have dropped over time from 50 to 10ppm), and in addition to the halimeda I have ulva and red macroalgae that grows on the rocks.
I was thinking, the only thing it could be is a testing error or I’m adding additional alk somehow and not realizing it. Well there it is — the additional alk is coming from carbon dosing and macros using nitrate. It’s not a huge spike, but has been a small increase that I continue to see add up over time, so this makes sense to me.
The halimeda being added just put additional strain on the calcium needs of the tank. I’m going to remove a bunch of that and see if it helps enough to resolve the issue.
I think there may be some confusion here.
The nitrate decline from 50 to 10 ppm would have boosted alk by about 1.8 dKH, but if the nitrate now remains steady (regardless of level), there's no impact to alkalinity from nitrate production (from ammonia) and consumption. Those two processes exactly cancel each other out with respect to alkalinity.
So unless you dose nitrate, I don't see this as a concern going forward.
It's still dropping, but I'm about to where I'd like to be so will be taking action to maintain it now.I think there may be some confusion here.
The nitrate decline from 50 to 10 ppm would have boosted alk by about 1.8 dKH, but if the nitrate now remains steady (regardless of level), there's no impact to alkalinity from nitrate addition and consumption.
So unless you dose nitrate, I don't see this as a concern going forward.
There are no good trace element mixes. There’s always a few elements that become a problem. Every tank is completely different and it doesn’t matter if you dose off Calcium or Alk consumption, something will either drift out of range or become depleted. This is why each element should be handled individually.I disagree with this statement in this general way. Most trace metals cannot be found by ICP-OES in "normal" concentrations. I suggest to pick out one "lead" element like for example nickel, bring it to low detectable concentrations like 2 or 3 ppb, and conclude that all other trace metals dosed with this product are also in appropriate concentrations for corals. This is what I expect from a good trace element mix.
In case of All-For-Reef calcium and alkalinity concentrations are the "lead parameters".
Like stated above, I think imbalances in the calcium alkalinity ratio may reflect the ratio in the salt used. If one parameter in the salt is higher or lower than in the tank, the complete system of interrelated parameters may shift.
For example, if alkalinity is lower in the tank than in the salt mix, also calcium may be lower in the tank than in the salt mix, and may finally be lower in concentration than desired.
Let me ask you this…how does AFR handle iodine, iron, maganese, chromium, cobalt, or vanadium?
- Joined
- Aug 24, 2016
- Messages
- 1,506
- Reaction score
- 2,301
Again I disagree.There’s always a few elements that become a problem. Every tank is completely different
1) 27 years of experience
2) Most trace metals like iron, manganese, copper, zink, nickel and cobalt are useful in relatively wide ranges since their speciation and uptake is regulated by the organisms. With regular supply and consumption a dynamic equilibrium forms and keeps the concentrations stable, regardless of minor swings in supply or consumption.
Most trace metals are co-precipitated and depleted by calcification. The biggest differences will be between the aragonite skeletons of corals and calcifying green algae and calcite structures of coralline algae.
How do you want to regulate these trace metals in a different way? All ICP-OES can find is when they are already in high concentrations or overconcentrations. Let the concentrations bottom out, trusting that it will be ok?
I just want to add for clarification that it will also tell you a 0. I did an ICP about 2 months ago and got back a reading of like 0.1 Iron. Hair Algae ate it all....All ICP-OES can find is when they are already in high concentrations or overconcentrations...
Randy Holmes-Farley
Reef Chemist
View Badges
Staff member
Super Moderator
Excellence Award
Expert Contributor
Article Contributor
R2R Research
My Tank Thread
- Joined
- Sep 5, 2014
- Messages
- 68,057
- Reaction score
- 64,480
I just want to add for clarification that it will also tell you a 0. I did an ICP about 2 months ago and got back a reading of like 0.1 Iron. Hair Algae ate it all.
To Hans-Werner's point, ICP-OES (aka ICP-AES) cannot detect the natural concentration of iron in surface seawater. Thus, none may stll be plenty.
Here's my comment from my own analysis of iron:
Iron (Fe). The natural iron level varies a lot with depth, but surface seawater may have only 0.006 µg/L. The Triton LOD = 0.3 µg/L. I dose iron, and when I dose it I boost iron to roughly 1-2 µg/L, which would be detectable. This sample was taken more than a week after the last iron dosing, and none was detected as it gets depleted in the meanwhile. I’ve not yet seen a Triton test result for a real aquarium sample that had detectable iron, but that doesn’t mean these tanks are necessarily deficient. Iron is also a case where the form is critical, and ICP cannot distinguish form. Binding to organic matter, for example, can alter the bioavailability of iron.
OK. BTW…27 years is an appeal to authority.Again I disagree.
1) 27 years of experience
2) Most trace metals like iron, manganese, copper, zink, nickel and cobalt are useful in relatively wide ranges since their speciation and uptake is regulated by the organisms. With regular supply and consumption a dynamic equilibrium forms and keeps the concentrations stable, regardless of minor swings in supply or consumption.
Most trace metals are co-precipitated and depleted by calcification. The biggest differences will be between the aragonite skeletons of corals and calcifying green algae and calcite structures of coralline algae.
How do you want to regulate these trace metals in a different way? All ICP-OES can find is when they are already in high concentrations or overconcentrations. Let the concentrations bottom out, trusting that it will be ok?
These elements are not stable and need to be dosed daily so that they are continuously available as they do not stay present long. Let’s take iodine for example. Most ICP’s I’ve seen with AFR show depleted levels and sometimes elevated levels which can be bad for a tank. This is a problem, because you are unable to fine tune that element. There’s not much control. You can only dose and trust what you’ve been given in the bottle and I’ll be willing to bet that most do not know the concentration.
Look at Iron and Manganese for example. What if you have a large refugium with Marco algae? Or what if somebody is running a ATS? Do you think that these elements in the bottles are not going to be quickly depleted?
I see elements like Fluoride & Boron get depleted very quickly…how do you address those? Or do you trust the bottle and hope they’re in normal ranges? Fluoride specifically needs to be loaded initially until it hits a saturation point which in most systems will never happen with AFR.
Last edited:
Thank you for this information.To Hans-Werner's point, ICP-OES (aka ICP-AES) cannot detect the natural concentration of iron in surface seawater. Thus, none may stll be plenty.
Here's my comment from my own analysis of iron:
Iron (Fe). The natural iron level varies a lot with depth, but surface seawater may have only 0.006 µg/L. The Triton LOD = 0.3 µg/L. I dose iron, and when I dose it I boost iron to roughly 1-2 µg/L, which would be detectable. This sample was taken more than a week after the last iron dosing, and none was detected as it gets depleted in the meanwhile. I’ve not yet seen a Triton test result for a real aquarium sample that had detectable iron, but that doesn’t mean these tanks are necessarily deficient. Iron is also a case where the form is critical, and ICP cannot distinguish form. Binding to organic matter, for example, can alter the bioavailability of iron.
True, but the good thing is that this is all fixing to change very soon. There’s already some things in process now.To Hans-Werner's point, ICP-OES (aka ICP-AES) cannot detect the natural concentration of iron in surface seawater. Thus, none may stll be plenty.
Here's my comment from my own analysis of iron:
Iron (Fe). The natural iron level varies a lot with depth, but surface seawater may have only 0.006 µg/L. The Triton LOD = 0.3 µg/L. I dose iron, and when I dose it I boost iron to roughly 1-2 µg/L, which would be detectable. This sample was taken more than a week after the last iron dosing, and none was detected as it gets depleted in the meanwhile. I’ve not yet seen a Triton test result for a real aquarium sample that had detectable iron, but that doesn’t mean these tanks are necessarily deficient. Iron is also a case where the form is critical, and ICP cannot distinguish form. Binding to organic matter, for example, can alter the bioavailability of iron.
Similar threads
- Replies
- 16
- Views
- 304
- Replies
- 3
- Views
- 227
- Replies
- 31
- Views
- 777
- Replies
- 5
- Views
- 199
- Replies
- 13
- Views
- 369
New Posts
-
AIO Build steveschuergers 90 gallon Goni heavy mixed reef.
- Latest: Reefing_addiction
-
-
-


















