- Joined
- Nov 11, 2018
- Messages
- 462
- Reaction score
- 158
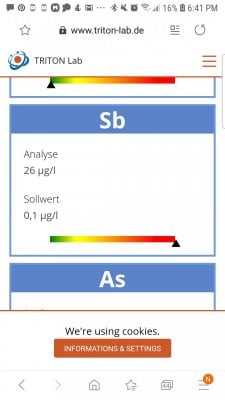
ok my antimony is thru the roof at 26 i am lost. help me please? ready ti do a 100% water change bc i am freqking out so bad.
is it possible that icp test was flawed? where could this have come from? will carbon remove?
is it possible that icp test was flawed? where could this have come from? will carbon remove?
Last edited by a moderator: