- Joined
- Sep 21, 2018
- Messages
- 6,685
- Reaction score
- 7,177
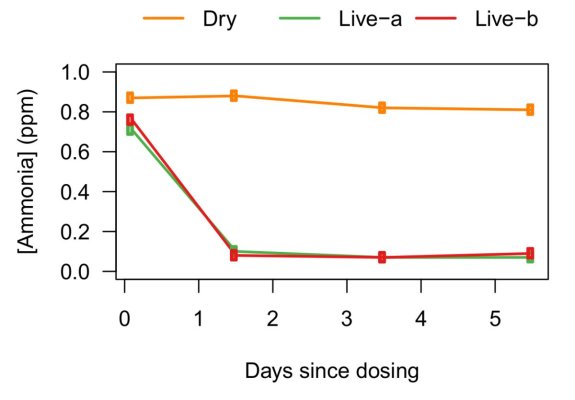
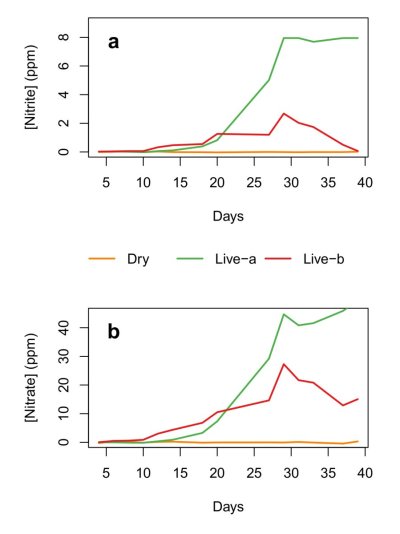
Just reread the Aquabiomics report and found similar experimental shortcomings that exist in the BRS experiment. The most serious is the lack of consistency of the organic and maybe even inorganic nitrogen concentration across the tanks. Like BRS, Aquabiomics rots live rock in the dark in the aquarium. While replications show similar effects in both replicates, the level of rotting biomass across experiments is not determined, nor is the algae accompanying each live rock sample taken into account. Another obvious missing or just unreported piece of information is concentration data for ammonia and nitrate throughout the experiment. It would have been very interesting to know what it was when livestock was added.Let's try to illustrate sixty's point here in a chart....
The below chart shows my subjective 1-10 "uglies score" at 15 weeks for each tanks, but split them into two groups.
The blue ones (first 7 listed) are ones that had photosynthetic material from lighted system that was placed directly into the test tanks.
The red bars (last 5) are the ones that either had no photosynthetic material, or who had a very extended dark cure time, or were never added to the lighted part of the tank (dark rubble).
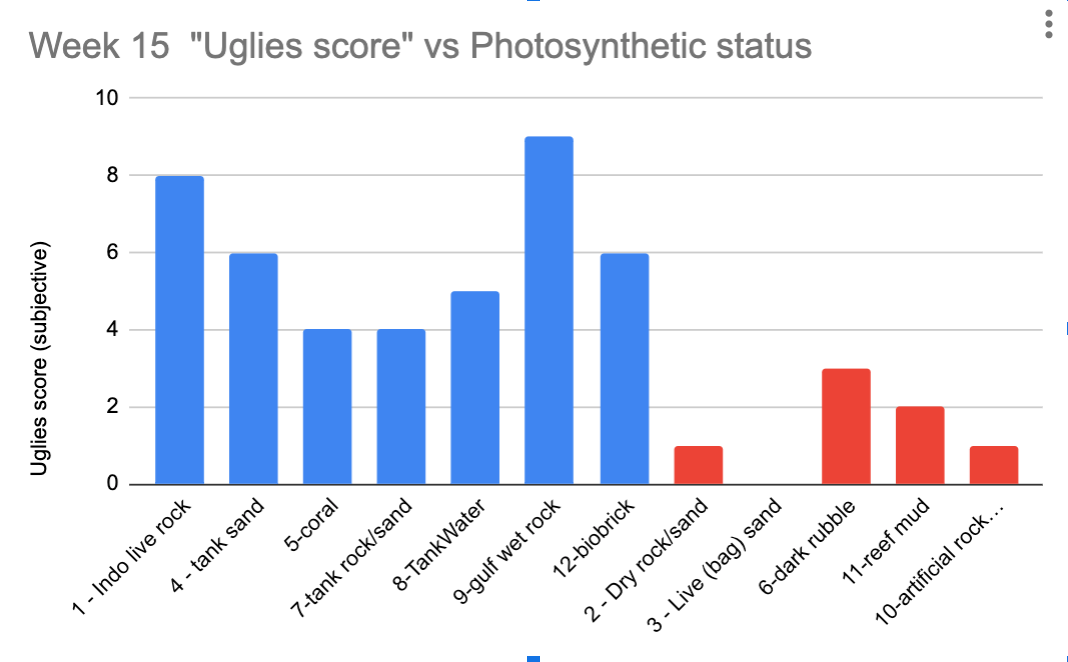
I share Ryan's conclusion here that I just wouldn't take lighted material from one system (or the ocean) and put it in a new system in a lighted portion. Not if I wanted to make life as easy as possible, anyway. In the absence of dedicated herbivores from the first week - it's sort of a foregone conclusion, like sixty said.
(I might point out here the similarities between the dark rubble treatment and the aquabiomics article here Establishing a Healthy Microbiome in a New Aquarium Using Live Rock. Interesting to see what results do get replicated.)
I am thinking right that nitrogen concentration played a role in the outcome of both Aquabiomics and BRS experiments, not biodiversity or balance. Also, I am thinking that we need to develop a new seeding method when using solid inoculum like live rock at least for conducting experiments if not for staring aquaria.