- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
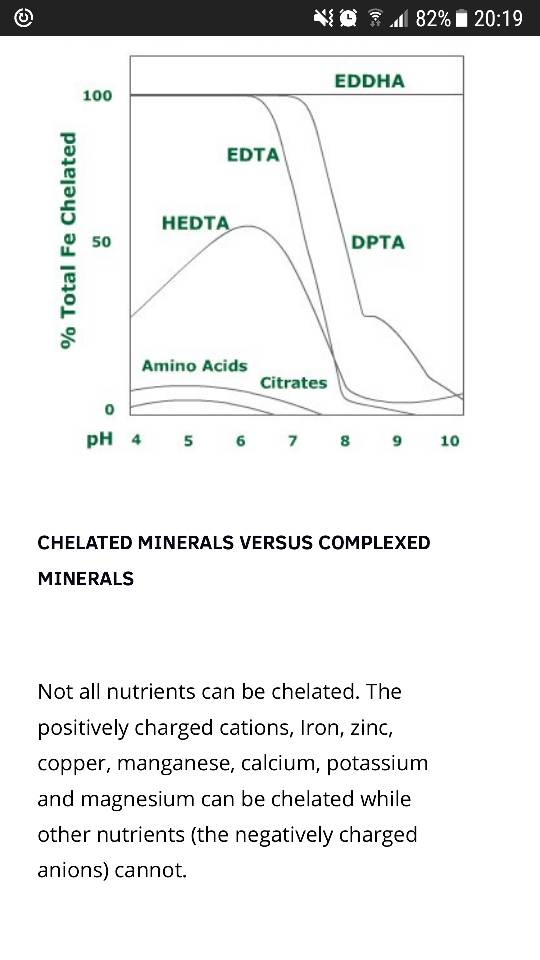
I wondered if anyone has a recommended dose for Fe and Mn (safe dose per liter aquariumwater in ppm). I am thinking about using Fe-EDDHA and a combination of manganese + trisodium citrate + vodka.
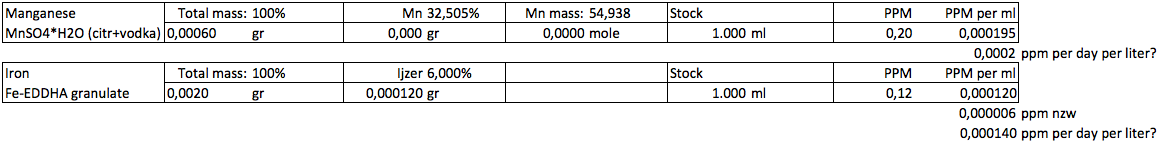
Would 0,0002 ppm (0,2 ppb) Mn and 0,00014 ppm (0,14 ppb) Fe be a good starting point (per liter aquariumwater)?
I do not know if a small amount of the chelated iron will remain stable in a large amount of water though.. the same goes for manganese. Else I will make it much more concentrated (like dosing a 0,1 ml for 100 gallons containing the total recommended dose)
Would 0,0002 ppm (0,2 ppb) Mn and 0,00014 ppm (0,14 ppb) Fe be a good starting point (per liter aquariumwater)?
I do not know if a small amount of the chelated iron will remain stable in a large amount of water though.. the same goes for manganese. Else I will make it much more concentrated (like dosing a 0,1 ml for 100 gallons containing the total recommended dose)