- Joined
- Aug 15, 2016
- Messages
- 285
- Reaction score
- 90
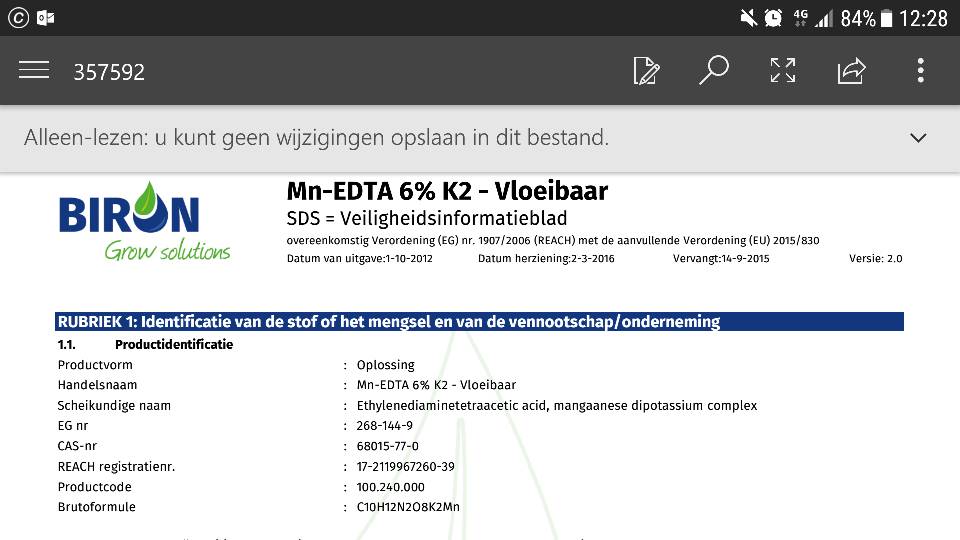
Today I received Mn-EDTA. it is 6%.. but is this by volume or weight?... 1 liter weighs 1.34 kG.. would it be 6 grams or 8.04 grams?
Apart from that.. it will be pretty concentrated stuff.. if it is by weight it wil contain 8040ppm. 1 drop is 0.38ppm (1/21 of a ml.. 1000ml is a bottle). so for my 400l tank.. 1 drop is 9ppb right? that is almost 5x the recommended amount..
If someone can confirm this, thank you!
Apart from that.. it will be pretty concentrated stuff.. if it is by weight it wil contain 8040ppm. 1 drop is 0.38ppm (1/21 of a ml.. 1000ml is a bottle). so for my 400l tank.. 1 drop is 9ppb right? that is almost 5x the recommended amount..
If someone can confirm this, thank you!