I have been having some weird chemistry issues in my tank the last couple of months after I dosed Chemiclean to rid a long lasting Cyano problem that I couldn't beat.
After I dosed Chemiclean the Cyano was was gone and has remained gone. I followed the directions to the T. My phosphates and nitrates have been great since as well. Here are my parameters.
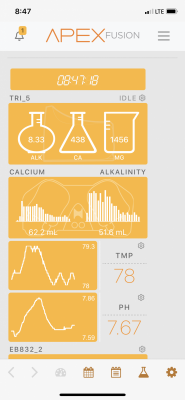
Alk- 8
Calcium- 420
Mag - 1450
Po4- .03-.06
Nitrates - 5-10
Ph - 7.6-7.8 or 8.1-8.2 (this is the weird one)
So since during Chemiclean my Monti's have not been good since. All other SPS looks good. My pH will fluctuate as low as 7.6 and I've seen it go up as high as 8.3. It will be higher a few days post weekly water change and then will drop over the week. My Alk consumption will follow that trend. My trident will dose up to 90ml a day but then today it is dosing 60ml because the pH has dropped. It's Tuesday and I do water changes on Thursday. I do 20% weekly water changes.
Prior to this my tanks natural pH was between 8.1-8.2. What could be causing all this instability? I keep hoping I can ride this out and the tank with stabilize itself, but it's been 2 months of this and not getting better. I have a co2 reactor I have hooked up and that doesn't seem to help much. I feel that I am doing everything I should be doing and this weirdness won't go away. My system is fairly simple just using skimmer, carbon, tons of flow, good lights, and water changes for nutrient export.
Anyone run into something like this?
After I dosed Chemiclean the Cyano was was gone and has remained gone. I followed the directions to the T. My phosphates and nitrates have been great since as well. Here are my parameters.
Alk- 8
Calcium- 420
Mag - 1450
Po4- .03-.06
Nitrates - 5-10
Ph - 7.6-7.8 or 8.1-8.2 (this is the weird one)
So since during Chemiclean my Monti's have not been good since. All other SPS looks good. My pH will fluctuate as low as 7.6 and I've seen it go up as high as 8.3. It will be higher a few days post weekly water change and then will drop over the week. My Alk consumption will follow that trend. My trident will dose up to 90ml a day but then today it is dosing 60ml because the pH has dropped. It's Tuesday and I do water changes on Thursday. I do 20% weekly water changes.
Prior to this my tanks natural pH was between 8.1-8.2. What could be causing all this instability? I keep hoping I can ride this out and the tank with stabilize itself, but it's been 2 months of this and not getting better. I have a co2 reactor I have hooked up and that doesn't seem to help much. I feel that I am doing everything I should be doing and this weirdness won't go away. My system is fairly simple just using skimmer, carbon, tons of flow, good lights, and water changes for nutrient export.
Anyone run into something like this?