I have a question on what the Redfield ratio actual is.
I understand the N : P ratio is 16:1 of Nitrogen : Phosphorus, not Nitrates : Phosphates. Although, I see them being used interchangeably in R2R and other sites.
Below, I did my best at showing the differences and how to convert Nitrate to Nitrogen and Phosphates to Phosphorus. So in this example I used Nitrates of 0.5ppm and Phosphates of 0.021ppm. This yields an N : P ratio of 16.5 and a NO3 : PO4 ratio of 23.8, which are significantly different.
Therefore, it appears the ratio is actually 23.1 if we're measuring Nitrates and Phosphates.
Also, this thread is not intended to be a thread on what Nitrate and Phosphate levels should be, but rather more of a chemistry question.
Thanks for any help on this!
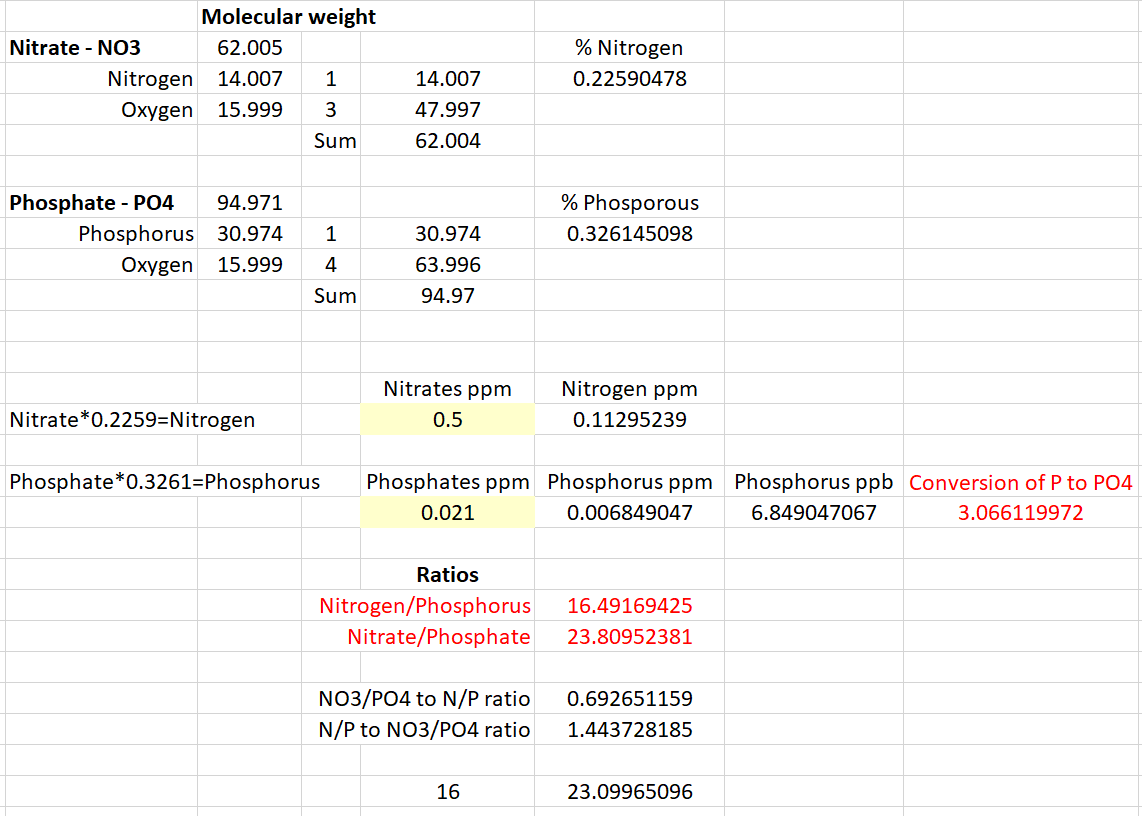
I understand the N : P ratio is 16:1 of Nitrogen : Phosphorus, not Nitrates : Phosphates. Although, I see them being used interchangeably in R2R and other sites.
Below, I did my best at showing the differences and how to convert Nitrate to Nitrogen and Phosphates to Phosphorus. So in this example I used Nitrates of 0.5ppm and Phosphates of 0.021ppm. This yields an N : P ratio of 16.5 and a NO3 : PO4 ratio of 23.8, which are significantly different.
Therefore, it appears the ratio is actually 23.1 if we're measuring Nitrates and Phosphates.
Also, this thread is not intended to be a thread on what Nitrate and Phosphate levels should be, but rather more of a chemistry question.
Thanks for any help on this!

















